Definition
In a confocal microscope, the illumination and detection optics focus on the same diffraction-limited point in the sample, which is the only point imaged by the detector during the confocal scan.
In order to generate a complete image, the spot must be moved over the sample, and data collected point by point. A distinct advantage of confocal microscopy is the provision of optical sectioning, which allows the 3D reconstruction of samples from high-resolution image stacks.
The primary function of a confocal microscope is to generate a point source of light and suppress out-of-focus light, enabling high-resolution imaging deep within the tissue and optical sectioning for 3D reconstruction of the imaged sample.
The basic principle of confocal microscopy is that the illumination and detection optics are focused on the same diffraction-limited point, which is moved across the sample to construct a complete image on the detector.
Although the entire field of view is illuminated during confocal imaging, anything outside the focal plane has little effect on the image, reducing the haze seen in thick and highly scattering samples in standard light microscopy and providing Optical sectioning.
Components of a Confocal Microscope
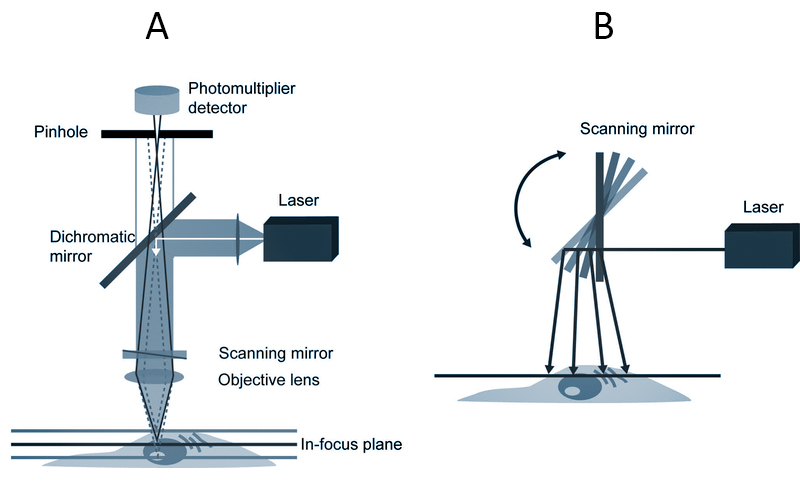
A. Light from a laser source passes through collimating optics to a variable dichroic mirror, or AOBS, and reflects an objective lens, which focuses the beam on a single point in the sample.
Scanning mirrors sweep the excitation beam point by point across the sample to construct an image. The emitted fluorescence passes back through the objective, dichroic mirror, or AOBS, and is detected by the PMT.
A pinhole placed in the conjugate image plane of the focal point in the sample is used to reject out-of-focus light, which is not picked up by the detector.
In this epi-fluorescence configuration, both illumination and emission light passes through the same lens, so only a pinhole on the detector side is required.
Changing the size of the pinhole changes the amount of light collected and the thickness of the optical section.
Spectral imaging can be achieved with an array of photomultiplier tubes and diffraction gratings or prisms placed in the emission path.
B. Schematic of a confocal microscope using a scanning mirror to sweep excitation light across a sample.
How Confocal Microscopy Works
Confocal microscopy works by point excitation in the sample (diffraction-limited points) and point detection of the resulting fluorescent signal. A pinhole in the detector provides a physical barrier to out-of-focus fluorescence. Only the focus or center point of the ventilated disc is recorded. Scanning the grating one sample at a time allows thin optical sections to be collected by simply changing the z focus. The resulting images can be stacked to produce a 3D image of the sample.
Types of Confocal Microscopes
- Laser Scanning Confocal Microscopy
- Spinning disk confocal microscope
- Hybrid Scanning Confocal Microscopy
Advantages of Laser Scanning Confocal Microscopy
Compared with traditional wide-field microscopes, laser scanning confocal microscopy has the characteristics of high definition, high resolution, and high sensitivity. It is widely used in the field of biomedicine and has become an important imaging tool in this field. What advantages make confocal get so much attention?
1. Only collect the signal on the focal plane to make the image clear from blur
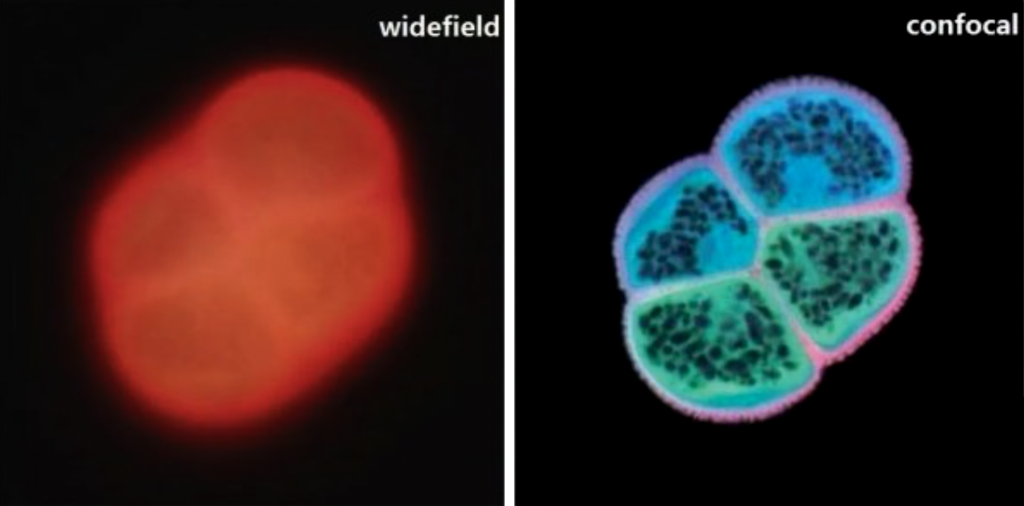
Figure 1 is the autofluorescence image of pollen grains. Because the sample is relatively thick, the general wide-field fluorescence microscope can only observe the overall sample structure, but the confocal microscope can perfectly display the sample outline and internal structure.
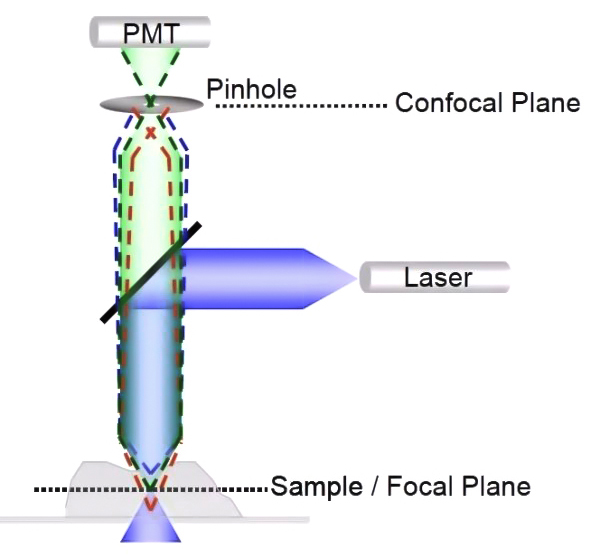
The confocal microscope realizes this advantage thanks to the key confocal hardware—the pinhole, as shown in Figure 2. It is a small hole in front of the detector and placed on the optical plane conjugate to the focal plane of the sample.
When the excitation light irradiates the sample, both the focal plane and the non-focal plane of the sample area will be excited to generate fluorescence signals, adjust the appropriate pinhole size (usually 1 Au), so that the fluorescence signal on the focal plane (green dotted line) can be Fluorescent signals (red and blue dashed lines) that pass through the pinhole to reach the detector out of the focal plane cannot reach the detector through the pinhole.
Through the addition of hardware pinholes, the confocal can obtain clear and high-quality images without changing the method of film production of ordinary fluorescence microscopes.
2. Continuously acquire signals of different focal planes to achieve three-dimensional reconstruction
Due to the existence of the pinhole, we can only acquire the signal on the focal plane and obtain a high-resolution two-dimensional image. By continuously adjusting the Z-axis position, two-dimensional images at different Z-axis positions can be obtained, and a series of continuous “optical slice” images can be obtained.
After obtaining these optical slices, the 3D module equipped with the instrument software can be used to reconstruct the 3D image to construct a clear 3D image. This non-destructive, continuous collection of optical slice images realizes the function of “cell CT”.
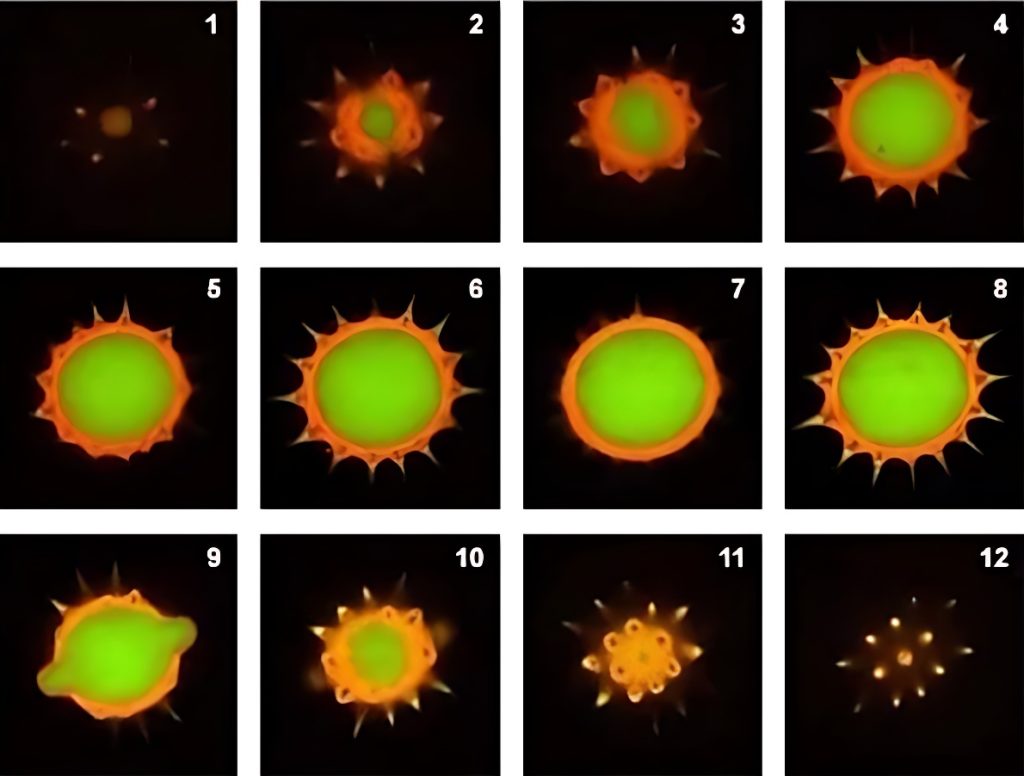
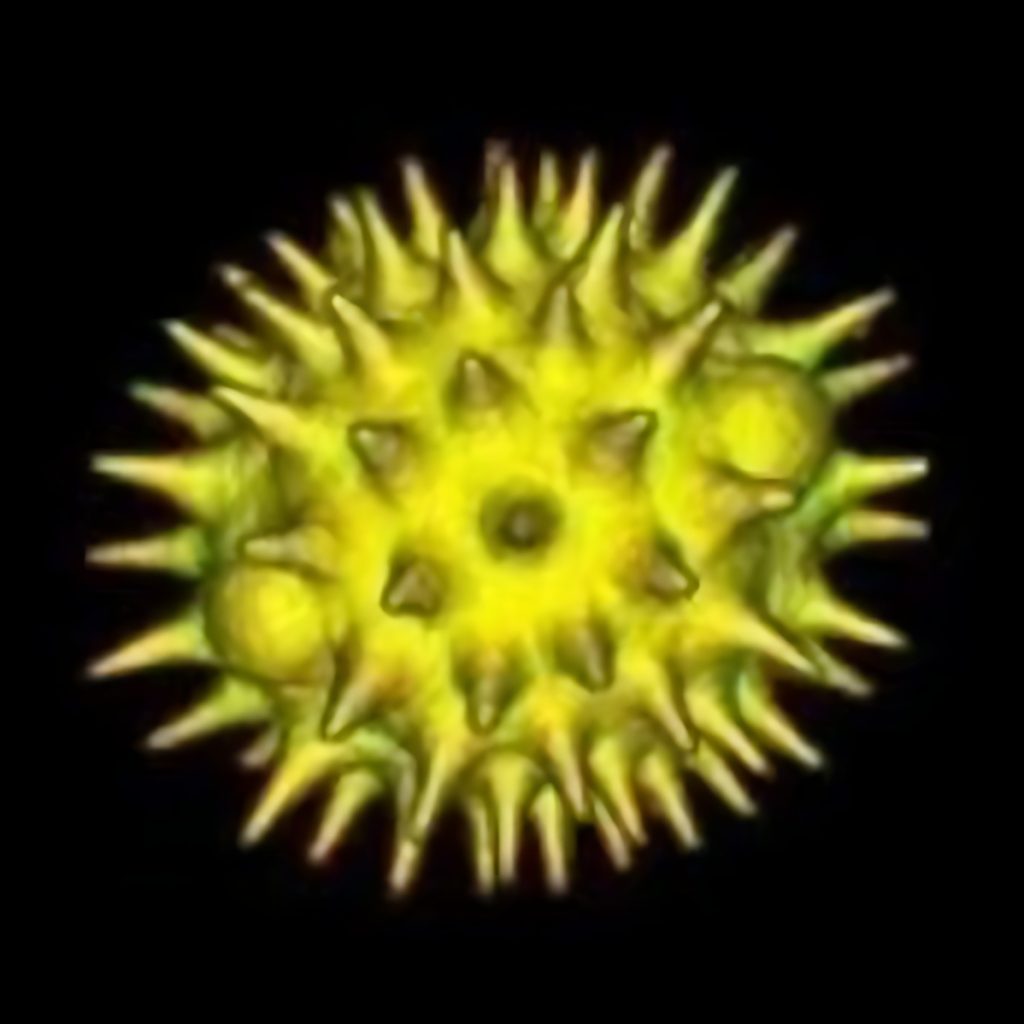
Below is a set of images from pollen grain autofluorescence optical sectioning sequence (commonly known as Z-sequence, Figure 3), and its three-dimensional composite 3D map (Figure 4).
3. Applied to Co-localization research
The accuracy of colocalization results is closely related to the resolution of the image, so we need to choose a higher-resolution imaging method when performing colocalization experiments. Compared with traditional microscopes, confocal microscopy has become a powerful tool for colocalization research due to its high-resolution advantages.
Due to the existence of the diffraction limit, the conventional confocal optical resolution is around 200nm. When we need to observe structures larger than 200nm, confocal can accurately determine the degree of colocalization.
If colocalization analysis is required, the colocalization analysis module equipped in the confocal software can be selected.
Light Sources for Confocal Microscopy
The basic components of a modern confocal microscope are the same pinhole, objective, and low-noise detector as in the original design, but often also include fast scanning mirrors, filters for wavelength selection, and laser illumination. The resulting light source is more stable, more uniform, generates less heat, and emits across a wide range of visible wavelengths.
Due to the light-repelling nature of confocal microscopes, detectors are still primarily high-sensitivity photomultiplier tubes (PMTs). These are essentially single-point cameras that maximize the light budget by amplifying the signal from the optoelectronic device.
