Lithium Ion Battery
Since the first commercialization of lithium-ion batteries (LIBs) in 1991, lithium-ion batteries have become one of the most popular energy storage devices since the 21st century due to their advantages such as high specific energy, long cycle life, no memory effect, and high safety. It has the advantages of high energy density, high single output voltage, excellent cycle performance, fast charge and discharge, and long service life. It is widely used in energy storage power systems for consumer electronics, electric vehicles, and new energy power stations.
After decades of development, China has become the world’s largest producer and consumer of lithium-ion batteries. According to the application field, lithium-ion batteries are mainly divided into energy storage batteries, consumer batteries and power batteries. The current demand in the field of consumer lithium-ion batteries has become saturated. With the development of the global new energy industry, new energy vehicles have gradually become a large-demand industry for lithium-ion batteries, which has promoted the rapid development of the power lithium-ion battery industry chain.
The lithium-ion battery is a secondary battery that mainly relies on the movement of lithium ions between the positive and negative electrodes to work. During the charging and discharging process, lithium ions intercalate and deintercalate back and forth between the two electrodes, and the energy storage and release of lithium ions are realized through the redox reaction of the electrode materials.
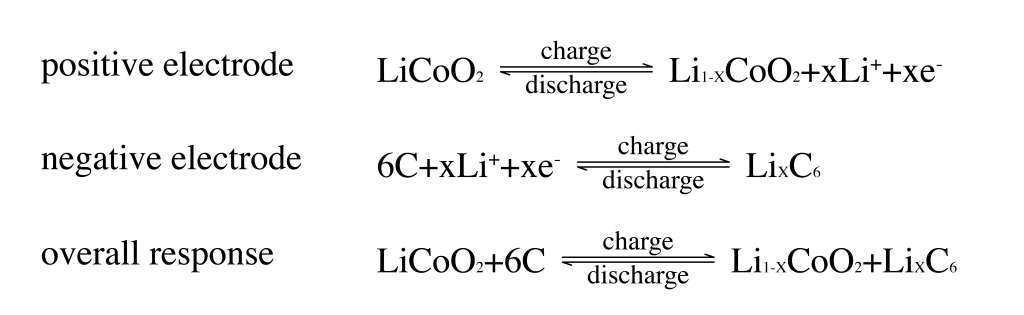
LIB is mainly composed of positive electrode material, negative electrode material, separator, electrolyte and shell. Among them, the positive electrode material is the main source of lithium ions, the negative electrode material is an important factor in providing specific capacity, and the separator provides a microporous channel for lithium-ion transmission. Its structure schematic diagram is shown in Figure 1. When charging, lithium ions (Li+) come out of the positive electrode, pass through the separator in the electrolyte, reach the negative electrode and embed in the negative electrode lattice. At this time, the positive electrode is in a lithium-poor state, and the negative electrode is in a lithium-rich state. ; while discharging, Li+ is released from the negative electrode in the lithium-rich state and then passes through the separator in the electrolyte to reach the positive electrode in the lithium-poor state and is inserted into the positive electrode lattice. At this time, the positive electrode is in a lithium-rich state and the negative electrode is in a lithium-poor state.
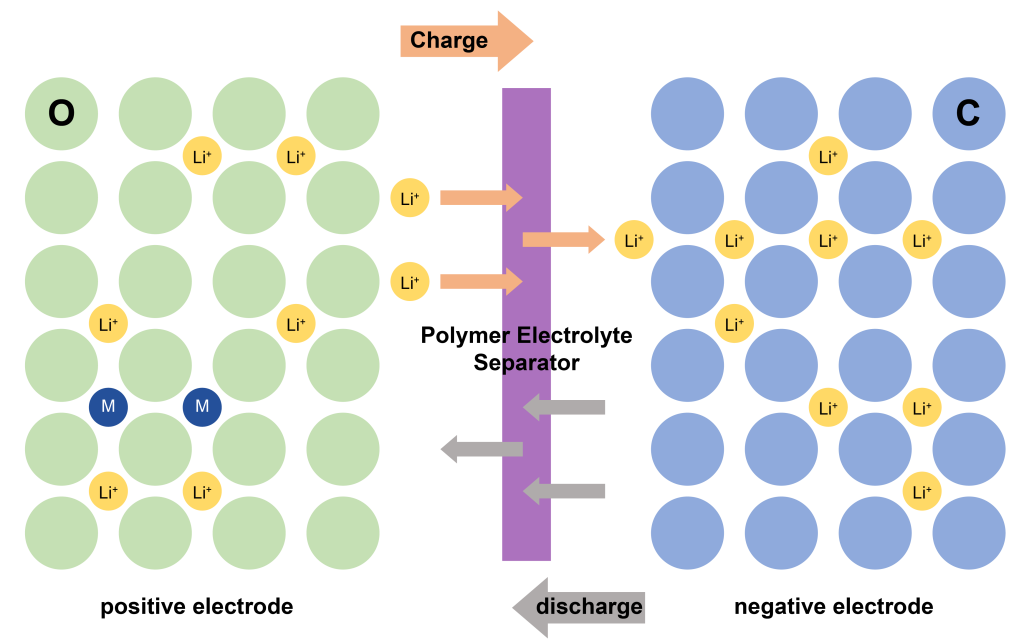
The scanning electron microscope independently developed by the company can quickly and reliably detect materials such as positive electrode materials, negative electrode materials, and separators in the field of lithium-ion batteries to avoid battery failure caused by low-quality raw materials, introduction of impurities, and improper processing techniques. Facilitate the in-depth research of lithium battery materials, and then improve the performance of lithium-ion batteries from all aspects.
Application of Scanning Electron Microscope in Lithium Battery Cathode Materials

The positive electrode material is the “lithium source” in lithium-ion batteries. It usually not only provides lithium ions that travel back and forth between the positive and negative electrodes during charging and discharging, but also provides the solid electrolyte phase interface (SEI for short) formed by the first cycle of charging and discharging of lithium-ion batteries. ) The lithium ions consumed by the negative electrode during the film. The power of the battery is affected by many factors such as the structure of the positive electrode material, doping modification, surface coating and preparation process. The development of cathode materials with the advantages of safety, economy, high performance, and large capacity will effectively promote the wide application of LIBs.
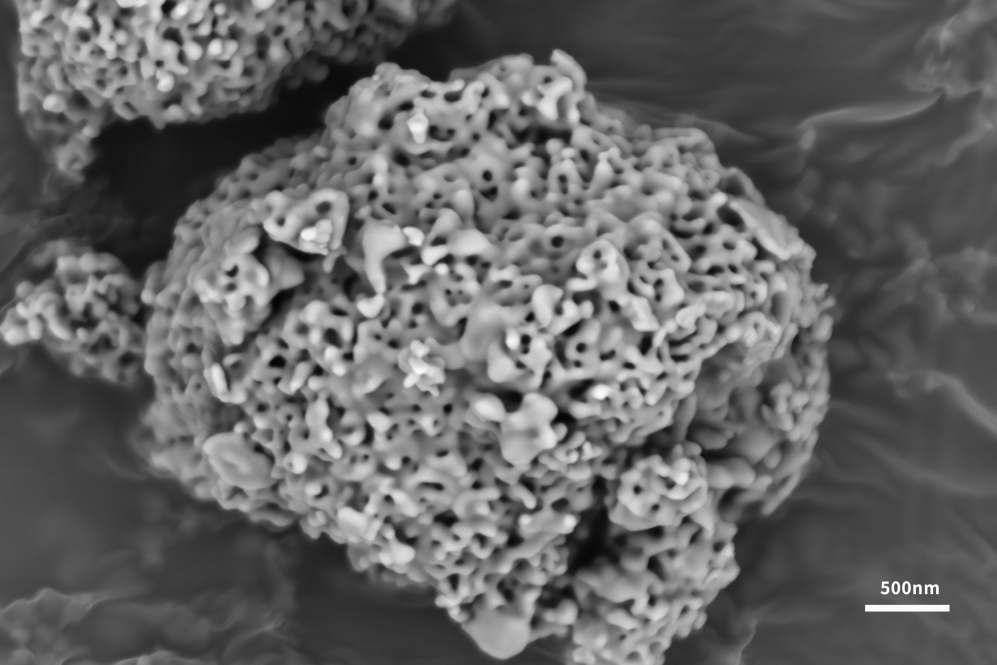
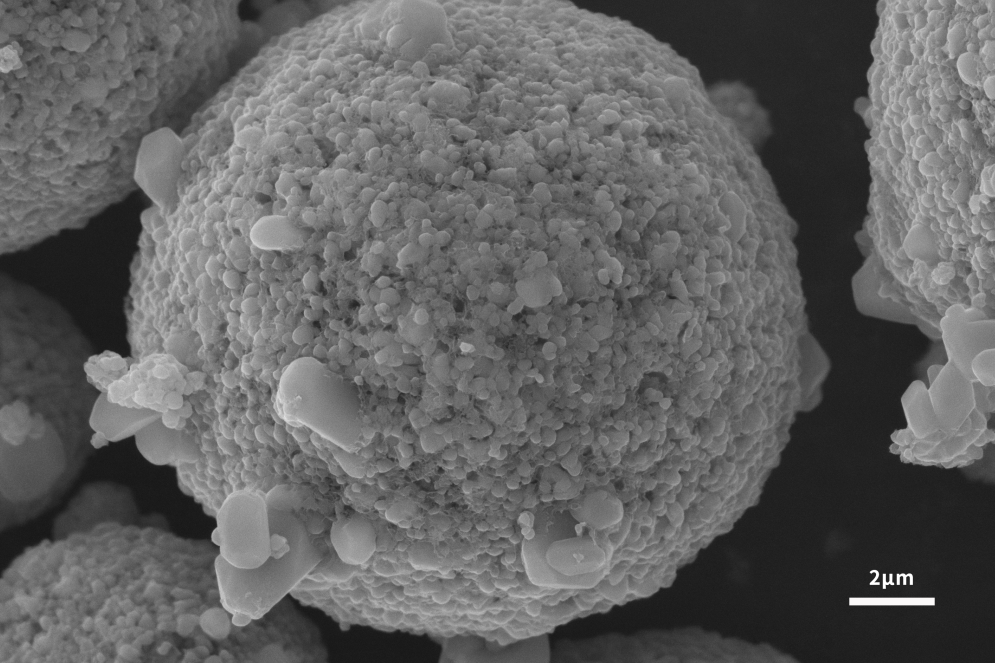
As shown in Figures 2 and 3, the scanning electron microscope can not only analyze the particle size and photograph the overall morphology of the slurry and the electrode sheet of the cathode material, but also provide powerful conditions for in-depth research and exploration of a specific cathode material system. A scanning electron microscope can be used to detect the distribution of positive electrode active materials, the degree of uniformity and dispersion of conductive additives on the positive electrode material after sizing and the surface of the electrode sheet after coating and rolling.
In addition, the single-particle morphology and particle distribution of the cathode material and its precursor can be characterized with the help of a scanning electron microscope. According to the results presented by the scanning electron microscope, it can help the design and improvement of the cathode material, and greatly improve the structural stability of the material and the performance of LIB.
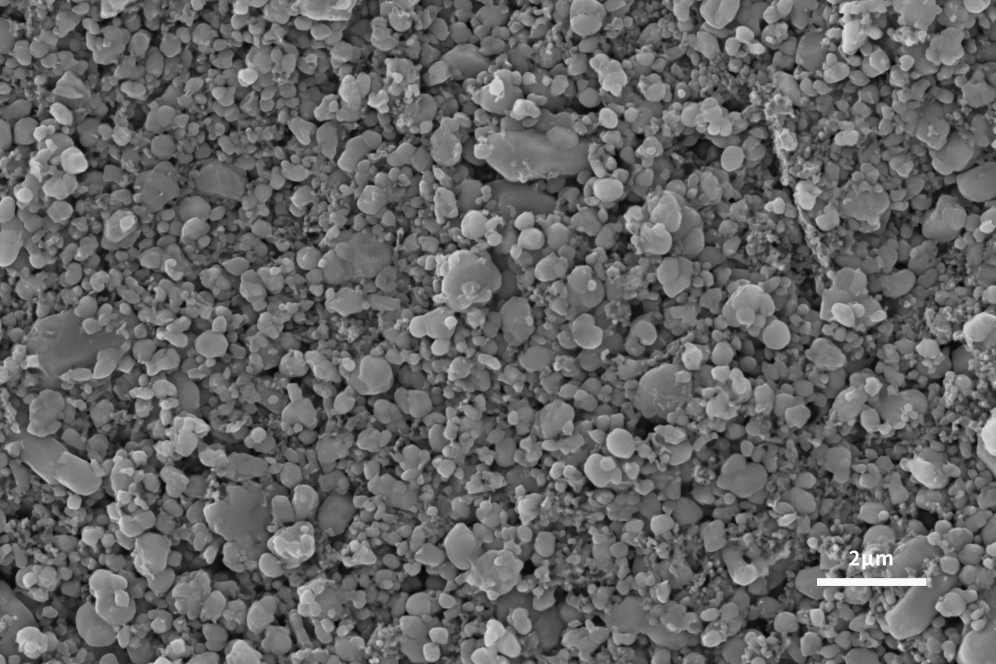

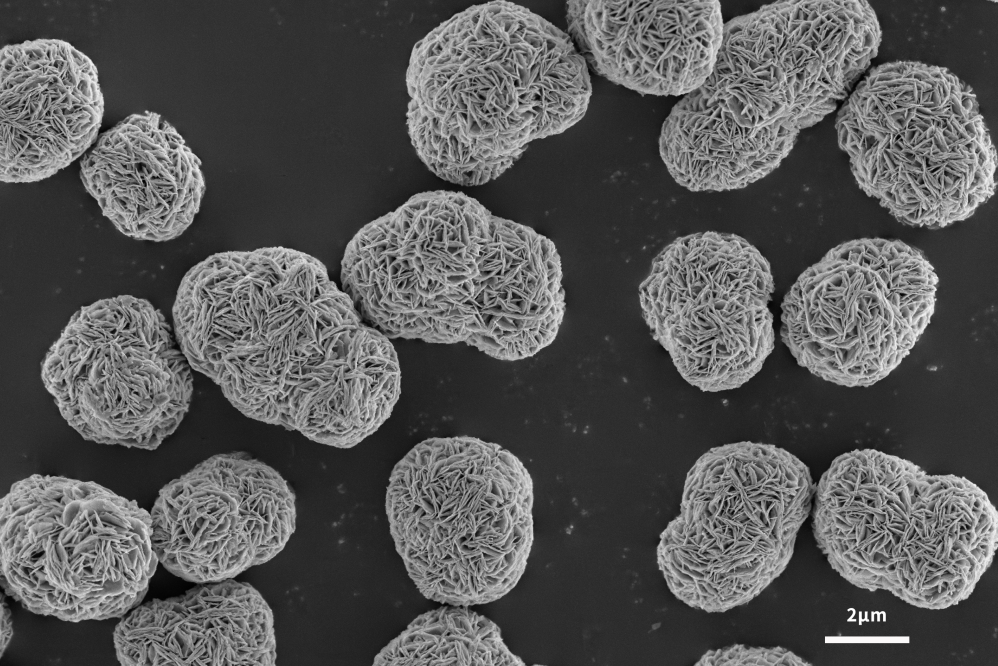

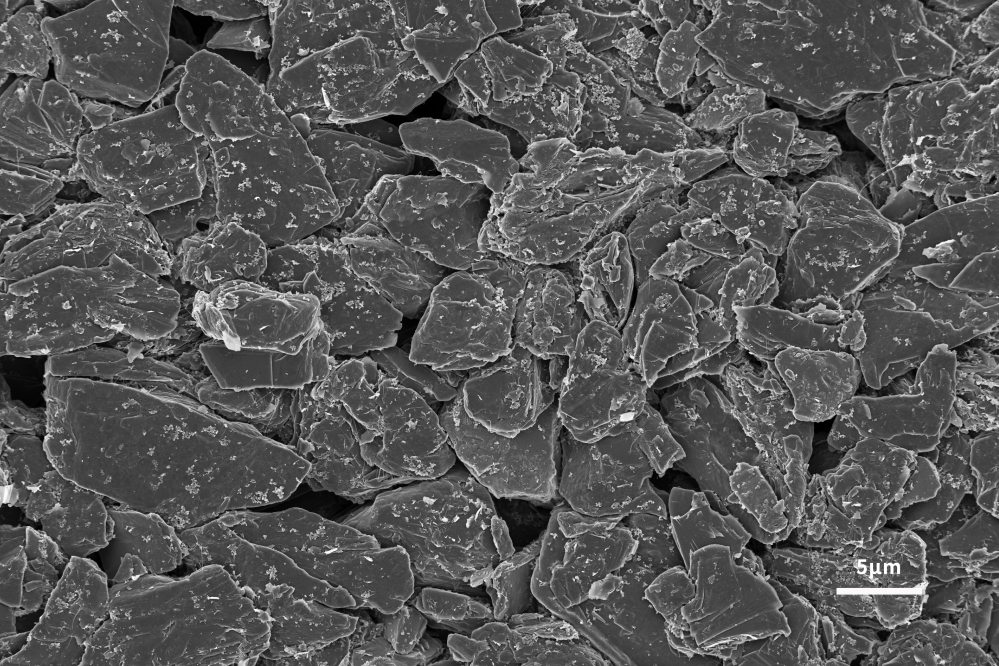
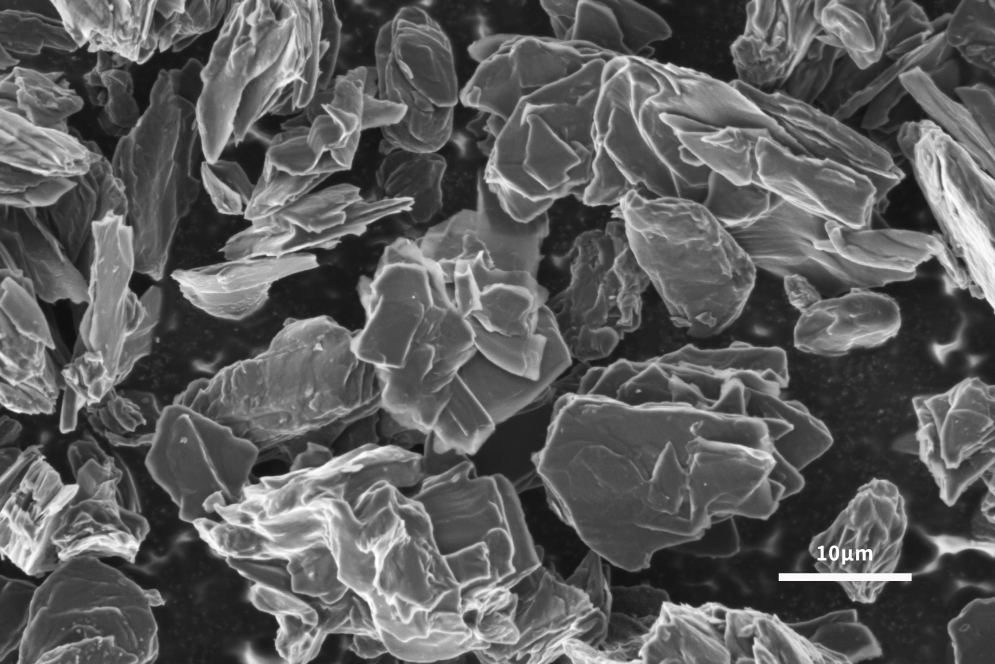
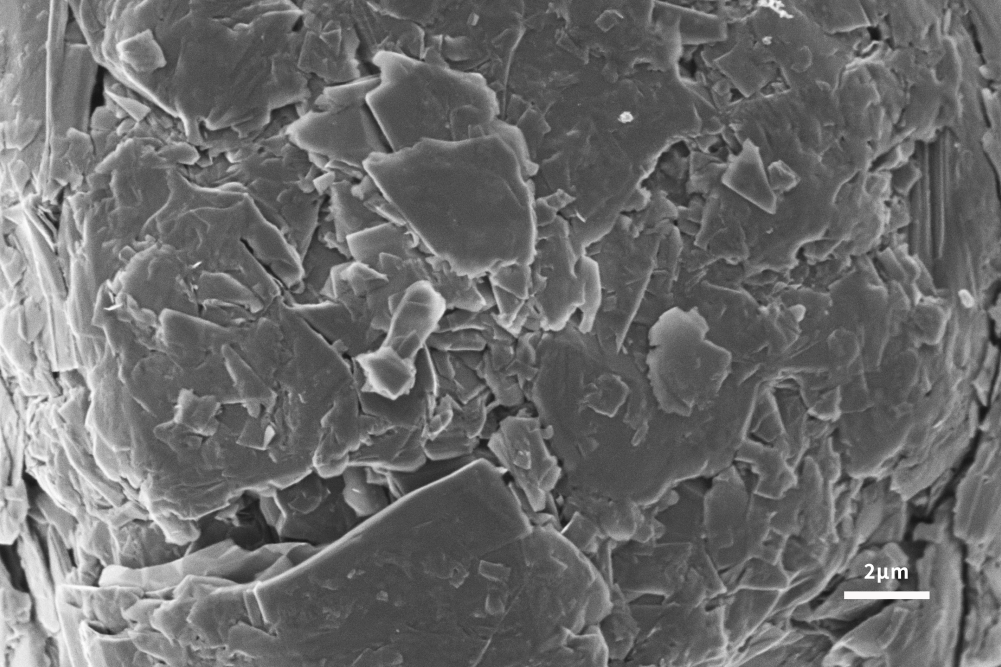
Application of Scanning Electron Microscope in Lithium Battery Anode Materials
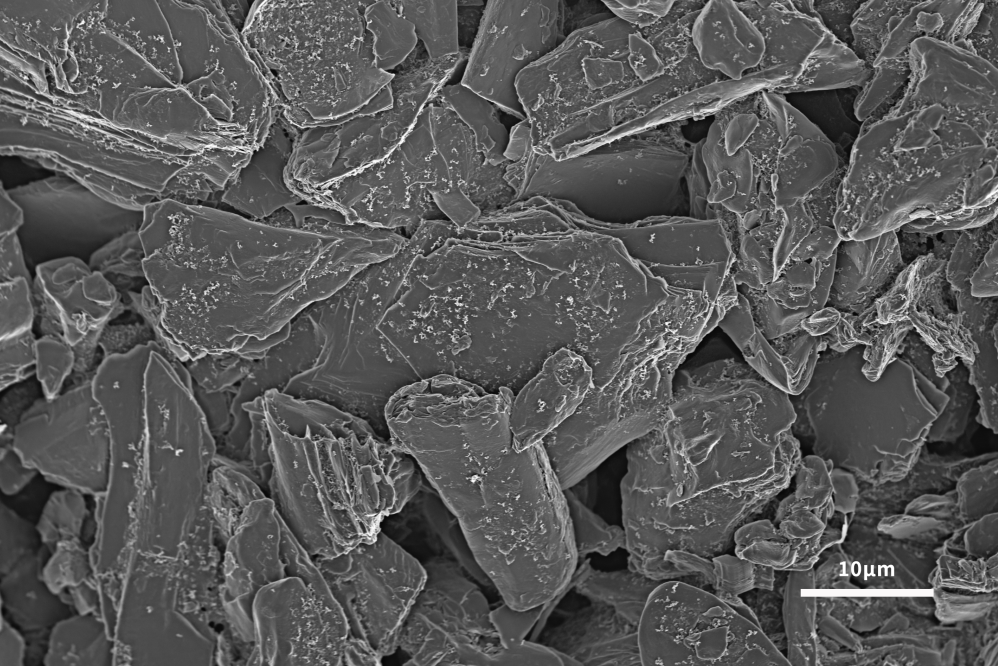
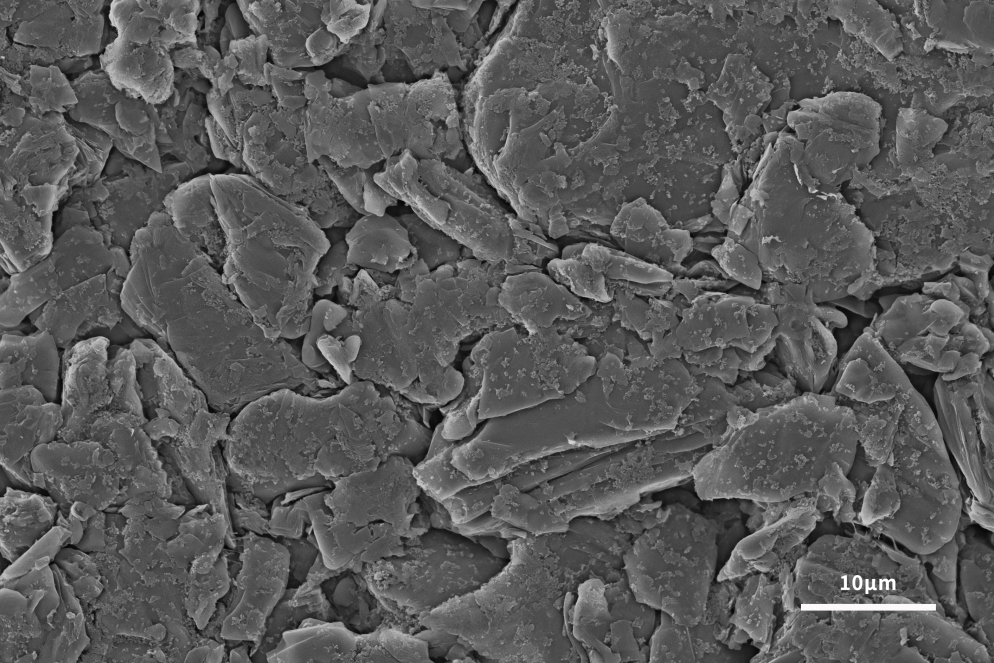
The lithium-ion insertion capacity of the negative electrode is the main factor determining the performance of lithium-ion batteries. In order to follow the development of advanced cathode materials, it is necessary to develop high-capacity anode materials to improve the performance of the whole lithium-ion battery. Graphite has been used as the main anode material since its commercial production in 1991. Graphite has the advantages of low cost, non-toxicity, repeated recycling and structural stability. It can be seen from Figures 4 and 5 that the scanning electron microscope can analyze the surface morphology of the slurried material and the coated pole piece, and at the same time analyze the size and shape of the graphite negative electrode to help explain the LIB performance differences caused by different graphite negative electrodes. . The lamellar structure morphology of graphite surface can be clearly observed by scanning electron microscope.
Application of Scanning Electron Microscope in Lithium Battery Diaphragm
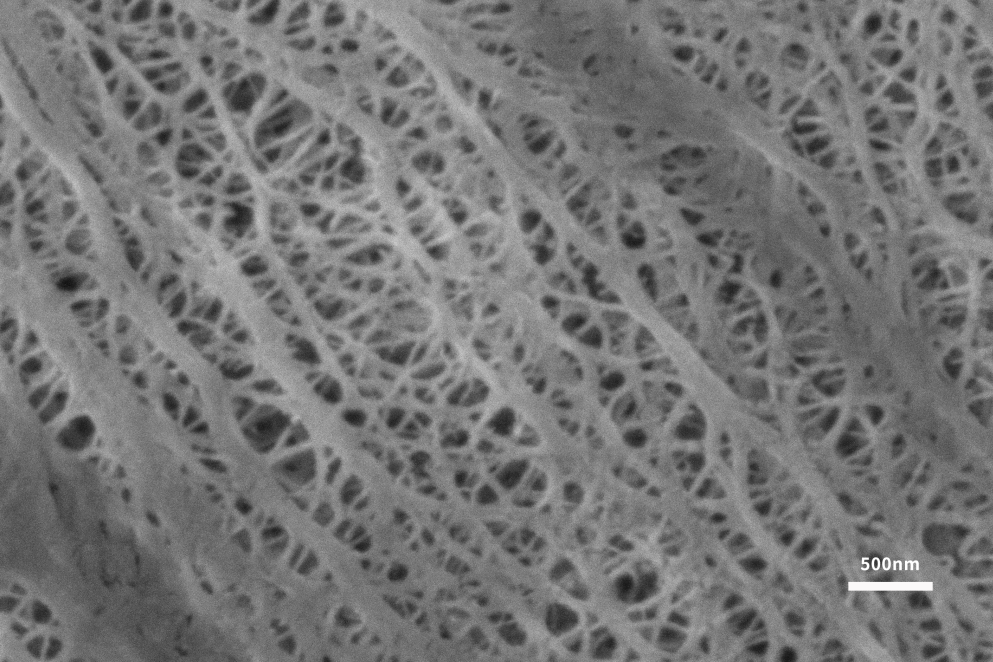
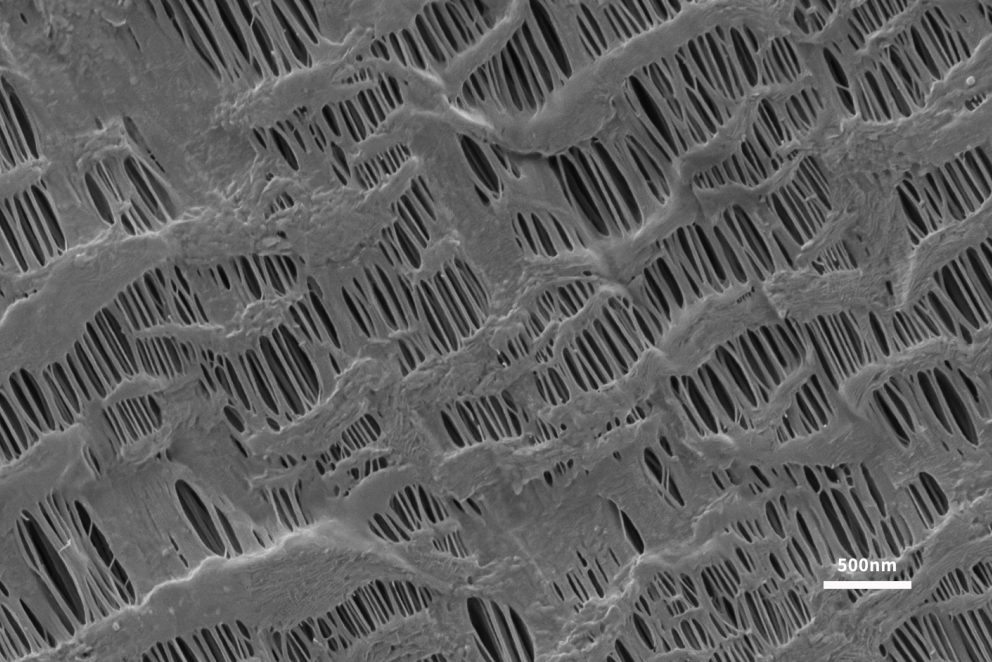
As a key material for lithium batteries, the separator plays the role of isolating electrons, which can prevent direct contact between the positive and negative electrodes and allow lithium ions in the electrolyte to pass freely. The separator plays a vital role in ensuring the safe operation of the battery [5]. At present, commercial lithium battery separators on the market are mainly microporous polyolefin separators based on polyethylene (PE) and polypropylene (PP). These polymer materials rely on low cost, good mechanical properties, excellent The advantages of chemical stability and electrochemical stability are widely used in lithium battery separators. Guoyi Quantum Scanning Electron Microscope can directly observe the fine structure of the diaphragm surface under low pressure, and the pore size and porosity analysis of the diaphragm can be carried out according to the topography images taken (Figure 6).
Cutting-edge solutions for analysis and testing of lithium battery materials
With advanced quantum precision measurement technology as the core, the company focuses on the main channel of scientific instruments, and has launched a series of high-end scientific instruments that are “what others have” and “what others have” and has launched systematic raw material testing for the lithium-ion battery industry. Analysis and product quality inspection program. The company’s self-developed high-end scientific instruments such as scanning electron microscope, specific surface and pore size analyzer, and electron paramagnetic resonance spectrometer can respectively detect the negative electrode material, positive electrode material, separator and other raw materials of lithium-ion batteries, so as to avoid low-quality raw materials, Battery failure caused by the introduction of impurities and improper processing technology.