In geometric optics, the so-called ideal optical system is an optical system that can form a perfect image and an ideal image with a sufficiently wide beam of light for each point in a sufficiently large space.
But in fact, since any optical system has a pupil that limits the light beam, the diffraction effect it brings cannot be eliminated, so the image of the point object formed by the optical system should be a diffraction image spot.
Naturally, this image spot is very close to the point image, because the pupil of the optical system is usually much larger than the wavelength of the light wave, so the diffraction effect is extremely small.
The imaging resolution of an optical imaging system refers to its ability to distinguish between two close points or object details.
The “image” formed by the optical system of a point object is a Fraunhofer diffraction pattern. In this way, for two very close point objects, their “images” (diffraction patterns) may be indistinguishable, so there is no way to distinguish two point objects. That is, there is a minimum object-space resolution distance that can be distinguished.
Optical diffraction limit
According to the Rayleigh criterion, the optical resolution of an optical microscope is limited by the optical diffraction limit. Its minimum resolution can be defined as the minimum distance between two point sources of equal brightness that can be distinguished by a microscope. Whether it is a wide-field microscope or a confocal microscope, a single-point light source will produce an airy disc on the focal plane after being converged by the lens group and the objective lens. According to the Rayleigh criterion, the optical diffraction-limited resolution of an optical microscopy imaging system can be expressed as:
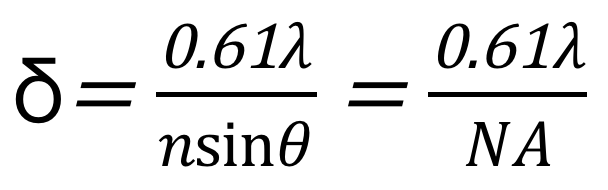
where λ is the wavelength of the incident light, n is the refractive index of the medium, θ is the half-aperture angle of the objective, and NA is the numerical aperture of the objective.
Suppose the wavelength λ of the light source is 400nm, the medium between the objective lens and the sample is air (refractive index n=1), and the resolution at this time is 200nm.
In order to improve the resolution of optical microscopes, early work often focused on how to reduce the size of the Airy disk, including reducing the wavelength of light and increasing the numerical aperture.
For the former, it directly led to the birth of various electron microscopes. Using electron beams with wavelengths of nanometers or even sub-nanometers for imaging, the resolution can reach nanometers or even smaller.
For the latter, on the one hand, a higher refractive index imaging medium can be used, such as immersing the sample in water to increase the refractive index in microscopic imaging. On the other hand, a more complex optical collection system (such as a conjugated double objective lens) can be used to increase the effective aperture angle of the optical microscope, thereby improving the relatively poor axial resolution of the optical microscope, a typical representative of which is the 4Pi microscope.
In addition, confocal pinholes can also be used to limit the size of the Airy disk and eliminate stray light, thereby improving the lateral and vertical resolution of the optical microscope, a typical representative of which is a confocal microscope (Confocal Microscope). It is worth pointing out that the optical microscope mentioned here refers to the far-field optical microscope (Far-field Optical Microscope), the distance between the objective lens and the sample is much larger than the wavelength of light.
Further studies have shown that the main reason for the resolution limit of the far-field optical microscope is that the far-field can only collect propagating wave signals, while the electric field strength of the evanescent wave (Evanescent Wave) carrying high-frequency information increases with the propagation distance. and decays exponentially.
Therefore, if the near-field optical signal is collected and detected using a probe only a few nanometers away from the sample surface, the resolution of the optical microscope can be greatly improved. This idea has led to the near-field scanning optical microscope (Near-field Scanning Optical Microscope, NSOM ), the resolution can be better than 25 nm. Optical microscopes based on evanescent field scanning imaging are often called near-field optical microscopes.
Super-resolution imaging
Optical microscopy has long been an important tool in biomedical research due to its non-contact and non-invasive advantages.
The resolution of ordinary optical microscopy imaging technology is sufficient for human cells with an average diameter of 10-20um.
Electron microscopy can achieve nanometer-level resolution and can observe the location of organelles such as vesicles and mitochondria inside cells. However, due to the lack of specific probe labels, it is not suitable for locating individual protein molecules, nor is it suitable for observing living cells and cell membranes. dynamic change process.
Therefore, biologists are eager to have an experimental microscopy method, which not only has submicron or even nanoscale optical resolution capabilities but also can continuously monitor the evolution of the microstructure of biological macromolecules and organelles without affecting the biological activity of biological systems.
Super-resolution optical microscopy came into being. Super-resolution optical imaging breaks the resolution limit of the optical microscope (in other words, it exceeds the resolution limit of the optical microscope, so it is called super-resolution optical imaging), increases the resolution from hundreds of nanometers to tens of nanometers, and provides life Scientific research provides unprecedented tools.
There are three main types of super-resolution microscopy
There are three main types of super-resolution microscopy, each of which works by a different mechanism. These include stimulated emission depletion (STED) microscopy, structured illumination microscopy (SIM), and stochastic optical reconstruction microscopy (STORM)/photoactivated localization microscopy (PALM).
Super-resolution microscopy has important applications in discovering new intracellular structures, biomolecular interactions, quantitative molecular counting, and dynamic time tracking.
1. Stimulated Emission Depletion (STED) Microscopy?
Stimulated emission depletion (STED) microscopy is a form of super-resolution microscopy that uses a technique called spatially patterned excitation.
In STED microscopy, two laser beams are used at the focal plane. When used together, excitation lasers and STED lasers reduce the effective point spread function (PSF). The lower the PSF, the higher the resolution.
STED lasers suppress excited fluorophores located near the excitation focus through the process of stimulated emission. The ring-shaped fluorophore around the center of the image field is suppressed, while the center is not. The result is a PSF below the diffraction limit. An extremely sensitive single-photon detector then receives the signal from the central fluorophore.
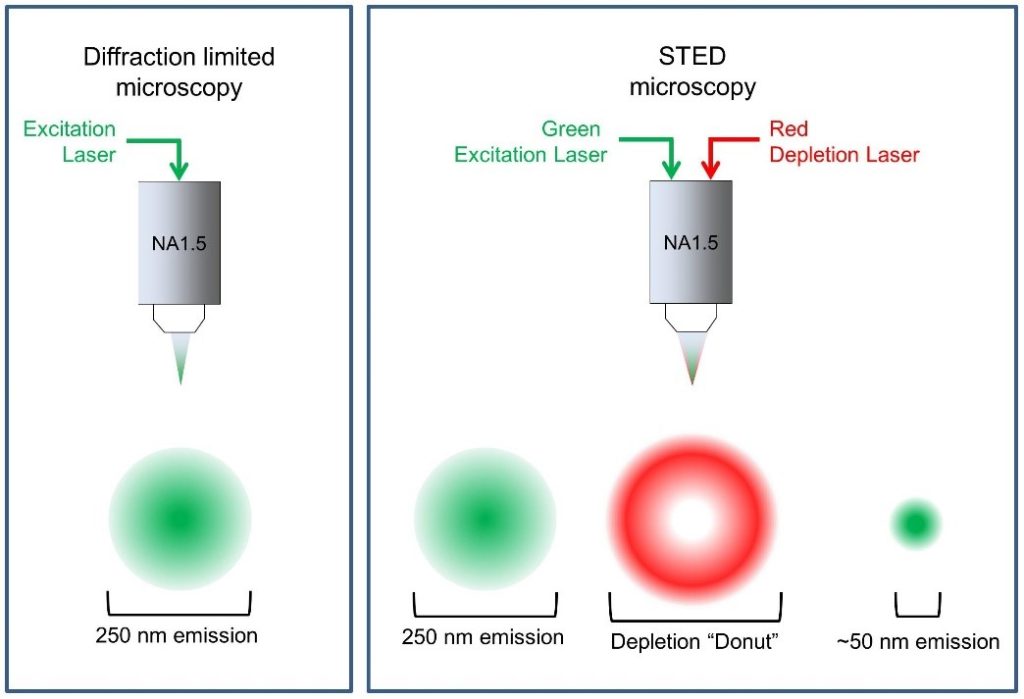
Saturated Structured Illumination Microscopy (SSIM) and Reversible Saturable Optically Linear Fluorescence Transition (RESOLFT) are other forms of super-resolution microscopy that use patterned excitation to increase resolution.
2. Structured Illumination Microscopy (SIM)
Structured illumination microscopy (SIM) is used to improve the spatial resolution of optical microscopy. Excite fluorescent samples multiple times using a stripe illumination pattern. The direction and position of the stripes change each time.
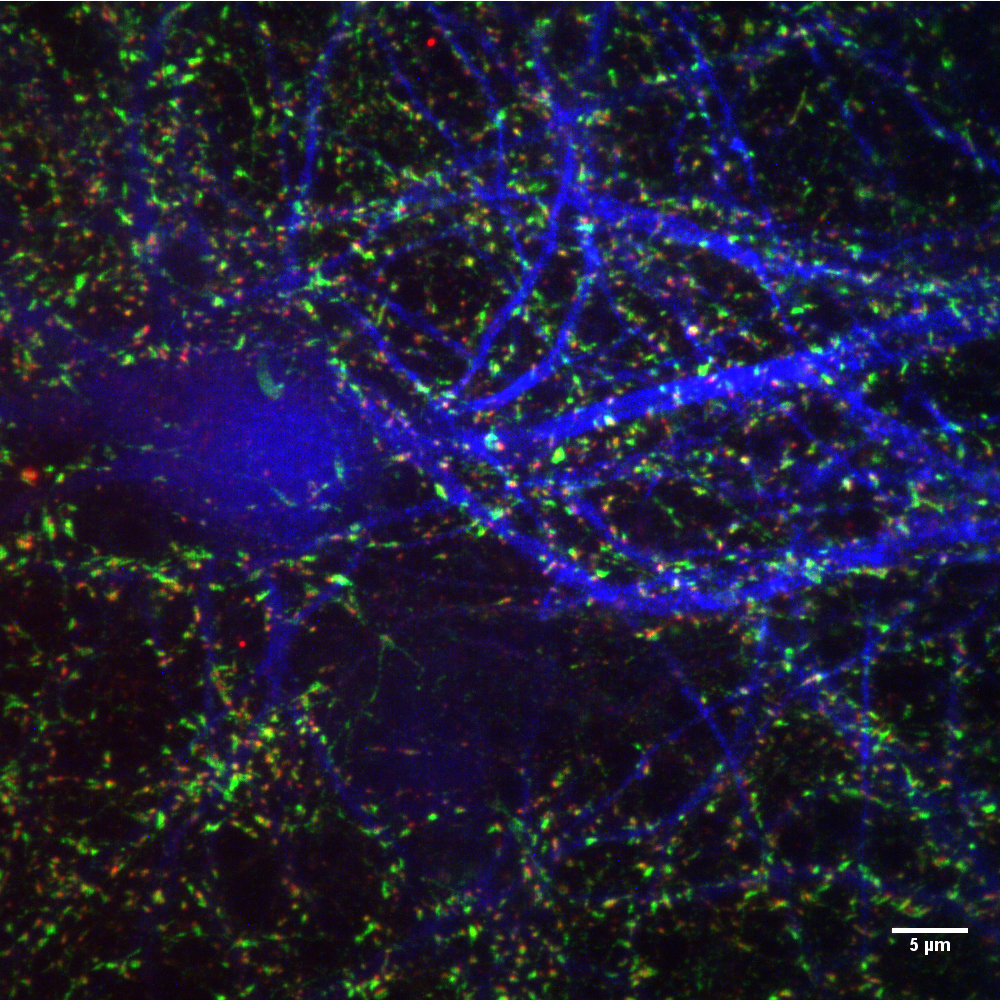
These images were analyzed using computer software that views moiré patterns. The fringes emitted towards the sample interact with the high-frequency light generated by the sample. This interaction produces a third pattern that can be analyzed more easily.
More detail can be obtained using multiple images, and the resolution of the reconstructed image is about twice that of conventional light microscopy.
Advantages of SIM include:
- Live cell imaging
- 3D imaging
- Thick slice imaging
3. Stochastic Optical Reconstruction Microscopy (STORM)
STORM is part of a family of single-molecule localization microscopy (SMLM) techniques that activate or excite only a small fraction of fluorophores at a time, reducing spatial overlap and allowing image resolution down to 5 nm.
STORM combines a photoswitchable dye with a lower-power activation laser that blinks or switches from a dark or off state to an emitting or on state. By imaging photoswitchable fluorophores over time, the precise location of these molecules can be identified. First introduced in 2006, STORM is one of the newer forms of super-resolution imaging.
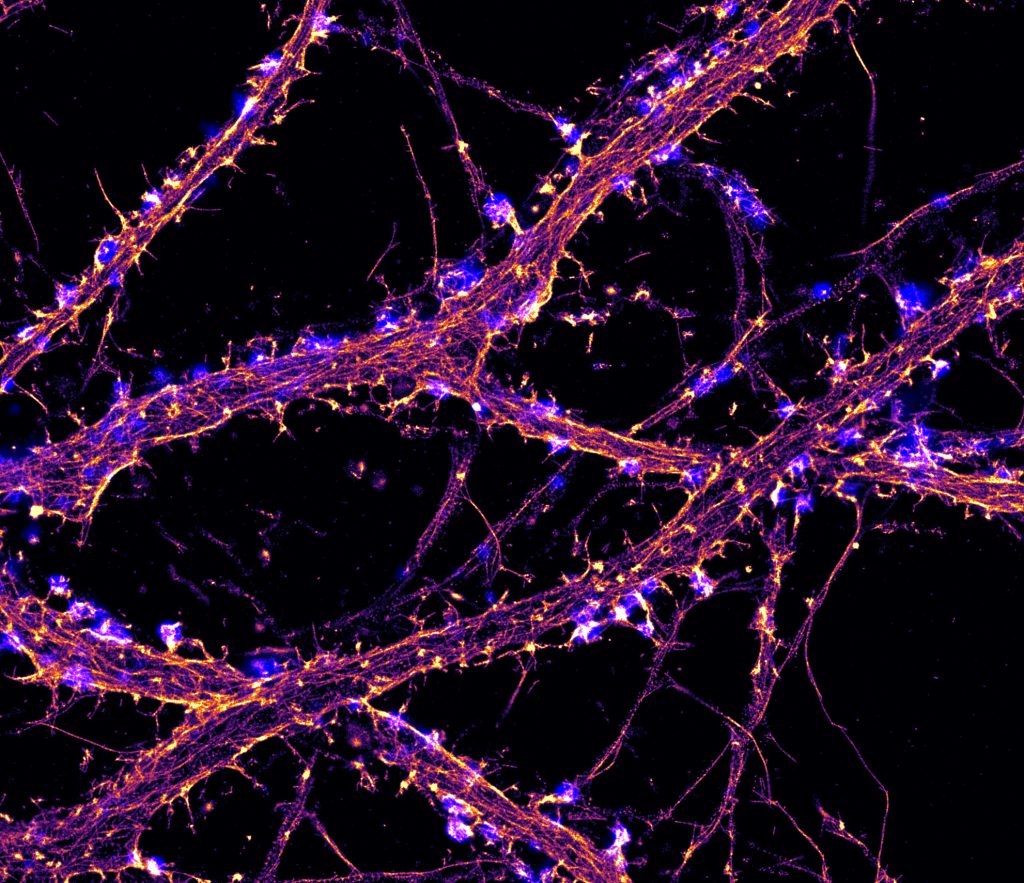
Photoactivated localization microscopy (PALM) and fluorescence photoactivated localization microscopy (FPALM) use a similar strategy to STORM.
4. dSTORM microscope
There are two types of STORM. The first type of STORM uses activator dyes and reporter dyes. The activator dye turns on the fluorophore and the reporter dye produces a signal. The second type of STORM, direct STORM (dSTORM), does not require an activator dye. During dSTORM, commonly used fluorophores can be combined with specialized buffers and laser light to induce photoswitching.
5. Two-photon excitation (TPE)
TPE is an optical process that uses multiphoton absorption to image living samples with less phototoxicity than conventional confocal microscopy.
Two-photon microscopy uses infrared excitation light to penetrate further into tissue, with minimal scatter compared to longer wavelength excitation light. For each excitation of the fluorophore, two photons of infrared light are absorbed, and the excitation is limited to a tiny focal volume, so the contribution of out-of-focus fluorescence is small.
Development trend
The development trend of ultra-high resolution microscopic imaging technology should be to meet the needs of three-dimensional, in vivo, and fast imaging in biological, material, and medical imaging, that is, to obtain higher spatial and faster time resolution to meet the smaller scale and faster The study of biological processes, and the need for deeper imaging depth to meet the imaging needs of tissues and individuals. Improvements should be made in the following aspects:
1. Develop new super-resolution hardware technologies: such as lasers with higher output power, lens optical components with lower optical loss, more stable optical systems, faster-scanning elements, microscopes with higher numerical aperture and longer working distance Objective lenses, faster and more sensitive single-photon detectors, smarter adaptive optics, and more.
2. Develop and optimize new probes and their labeling methods:
Structural analysis and modification of fluorescent proteins and dyes to improve the brightness, photostability, photoactivation, phototransformation, photoswitching characteristics, and conversion rates of fluorescent proteins and organic dyes;
Develop new organic and inorganic probes, such as gold particles, semiconductor quantum dots, polymer quantum dots, upconversion nanoparticles, etc.;
Improving the labeling specificity of probes and reducing non-specific binding are also efforts to improve resolution.
3. Development of multi-modal fusion: Combining fluorescence-based ultra-high resolution imaging technology with particle-based electron microscopy, taking advantage of their respective complementary advantages, and realizing the respective advantages of electron microscopy and optical microscopy, that is, ultra-high resolution and The perfect combination of molecular specificity is “correlative light & electron microscopy (CLEM)”.
4. Develop new data processing algorithms: In the face of three-dimensional large-scale imaging (such as the entire neuron, the entire brain tissue) and the demand for 4D genome imaging, we will face the processing of terabytes of data. The era of big data requires more efficient Image processing algorithms and software, in addition, deep mining of super-resolution data is also one of the future development directions.