Abstract: Optical microscopes, invented in the 17th century, have long been limited by optical diffraction in terms of resolution. In the 21st century, various super-resolution imaging technologies have revolutionized microscopy by overcoming the diffraction limits. Among them, nanoscale-resolution optical imaging based on single-molecule localization has achieved breakthroughs in recent years, reaching resolutions close to the molecular scale. This article provides a brief overview of the development of optical microscopes, and the basic principles of super-resolution imaging technologies, and focuses on the emerging nanoscale resolution microscopy and its potential applications.
I. Introduction to nanometer resolution optical microscopy imaging technology
Human perception relies heavily on visual information, constituting about 80% of the total information obtained from the natural world. However, the human eye’s resolution limit is around 100μm, roughly the diameter of a human hair. Traditional optical microscopes, developed in the 17th century by Leeuwenhoek and Hooke, allowed the observation of bacteria, cells, and their internal structures at the micrometer scale (Figure 1).
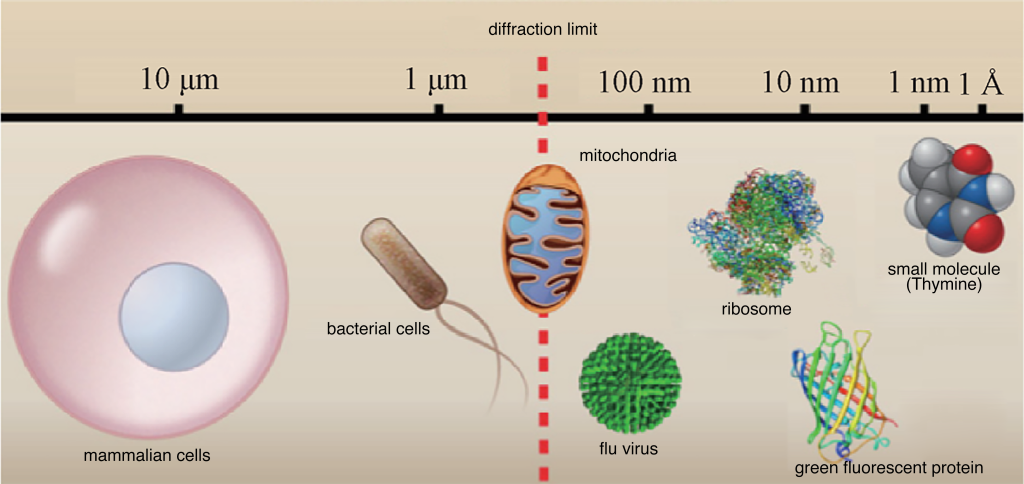
Figure 1: Comparison of different biological sample sizes with diffraction limit
Improving the microscope’s resolution and contrast is crucial to delving into the life activities of cells. Resolution, defined as the minimum distance between two distinguishable points, is essential for observing fine cellular structures. The diffraction limit restricts the minimum size of objects that optical microscopes can resolve. In the visible light range (400–700 nm), traditional optical microscopes’ lateral resolution is limited to 200–300 nm, and axial resolution is limited to 500–800 nm. This limitation hinders the study of subcellular structures in the nanometer to hundreds of nanometers scale.

Figure 2 The concept of diffraction limit (picture from Wikipedia)
In addition to resolution, contrast is also a vital indicator for microscopy. The development of phase-contrast and differential interference contrast microscopy in the 20th century significantly improved contrast, enabling detailed observations of transparent cellular structures. Fluorescence labeling and fluorescence microscopy techniques further enhanced contrast by marking specific structures or proteins within cells. Fluorescence imaging, with its high sensitivity, specificity, and resolution, provided powerful tools for studying subcellular structures and biomolecular functions.
II. Super-Resolution Microscopic Imaging Techniques
Since the beginning of the 21st century, various super-resolution fluorescence imaging techniques have been proposed, breaking the diffraction limits of optical microscopy. Three representative techniques are Stimulated Emission Depletion (STED), Structured Illumination Microscopy (SIM), and Single Molecule Localization Microscopy (SMLM).
STED, proposed by Stefan W. Hell in 1994, achieves super-resolution imaging by scanning a sample with both excitation light and a donut-shaped depletion light. The resulting effective imaging area is reduced below the diffraction limit, achieving resolutions in the tens of nanometers.
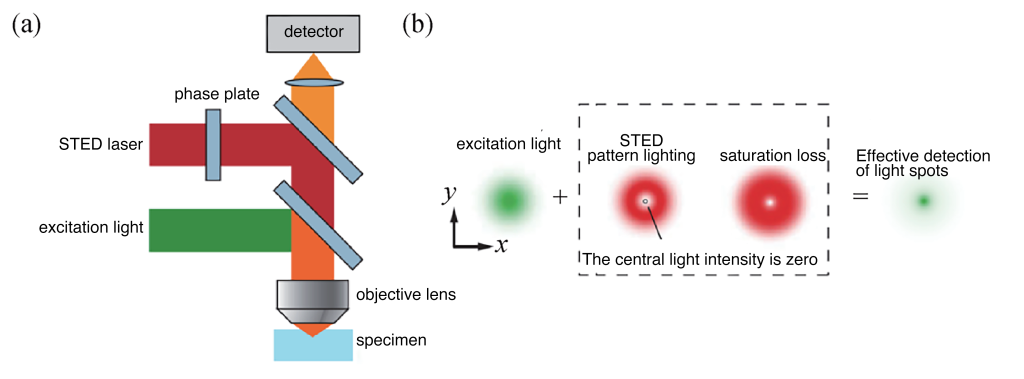
Figure 3 (a) Imaging optical path used in STED technology; (b) STED principle diagram
SIM, based on patterned illumination and camera detection, expands the spatial frequency range captured by the microscope by shifting high-frequency information to low-frequency regions. Through Moiré fringes, this technique allows the microscope to capture information beyond the traditional diffraction limit, improving resolution.
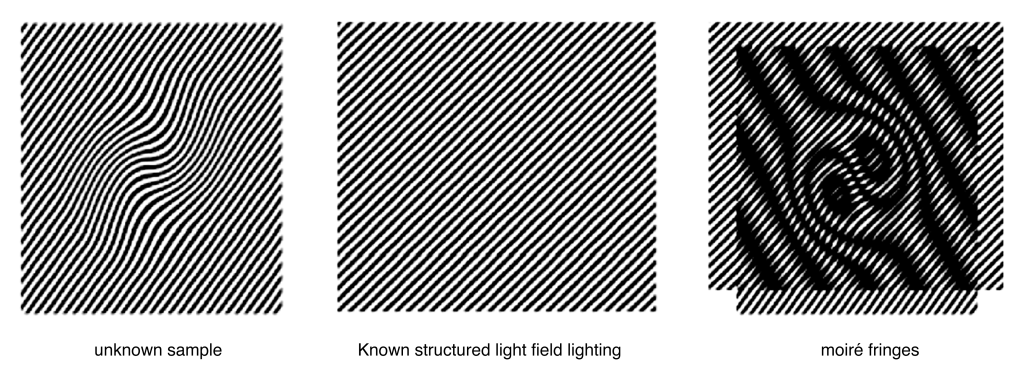
Figure 4 SIM technology principle. The high-frequency information of the unknown sample appears as thin stripes. When the unknown sample overlaps with the known structured light illumination pattern, the high-frequency information of the unknown sample is moved to low frequency and appears as wider stripes.
SMLM relies on the principle that the measurement error of a single molecule’s position can be much smaller than the size of its fluorescent spot. By reconstructing the positions of all fluorescent molecules in the sample, SMLM surpasses the diffraction limit, providing higher resolution for studying nanoscale cellular structures.
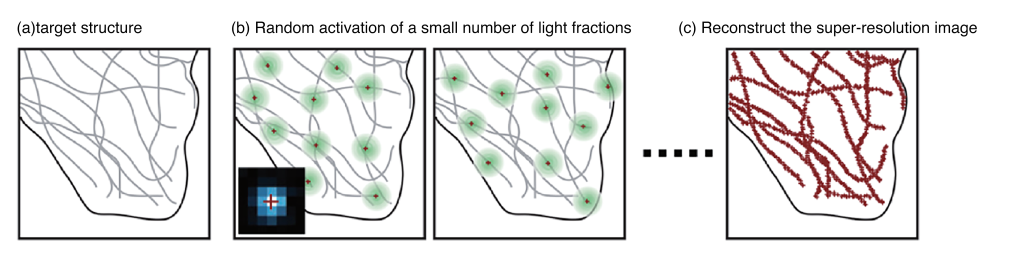
III. MINFLUX Microscopy Technology Combining STED and SMLM
MINFLUX microscopy, introduced by Stefan W. Hell in 2017, combines the principles of STED and SMLM. It creatively utilizes the donut-shaped light distribution of STED microscopy to excite fluorophores used in SMLM, ensuring only one fluorescent molecule is present in the excitation light field. This approach significantly enhances the spatial resolution by analyzing the relationship between the number of photons emitted and the light field, allowing precise determination of molecular positions.
IV. Single-Molecule Positioning Microscopy Based on Interference Fringe Excitation Technology
To improve the resolution of single-molecule positioning imaging while maintaining imaging speed and throughput, a team from the Institute of Biophysics, Chinese Academy of Sciences, developed the Interference Single-Molecule Positioning Microscopy (ROSE) technology. This technique utilizes interference fringes to excite fluorescent molecules, enabling higher precision in determining molecular positions.
4.1 Lateral Interference Single-Molecule Positioning Imaging
The lateral interference single molecule positioning imaging system is a two-dimensional positioning system. It requires six interference fringes for two-dimensional positioning (three for each direction: X and Y). In this system, the X-directional positions of single molecules are determined through three sub-images capturing the brightness information. By synchronizing the electro-optic modulator and the high-frequency resonant mirror, interference fringes are quickly generated and imaged (Figure 6(a)). Testing with high brightness and stable nanoscale fluorescent spheres demonstrated an approximately 2.4-fold improvement in positioning accuracy compared to centroid fitting.
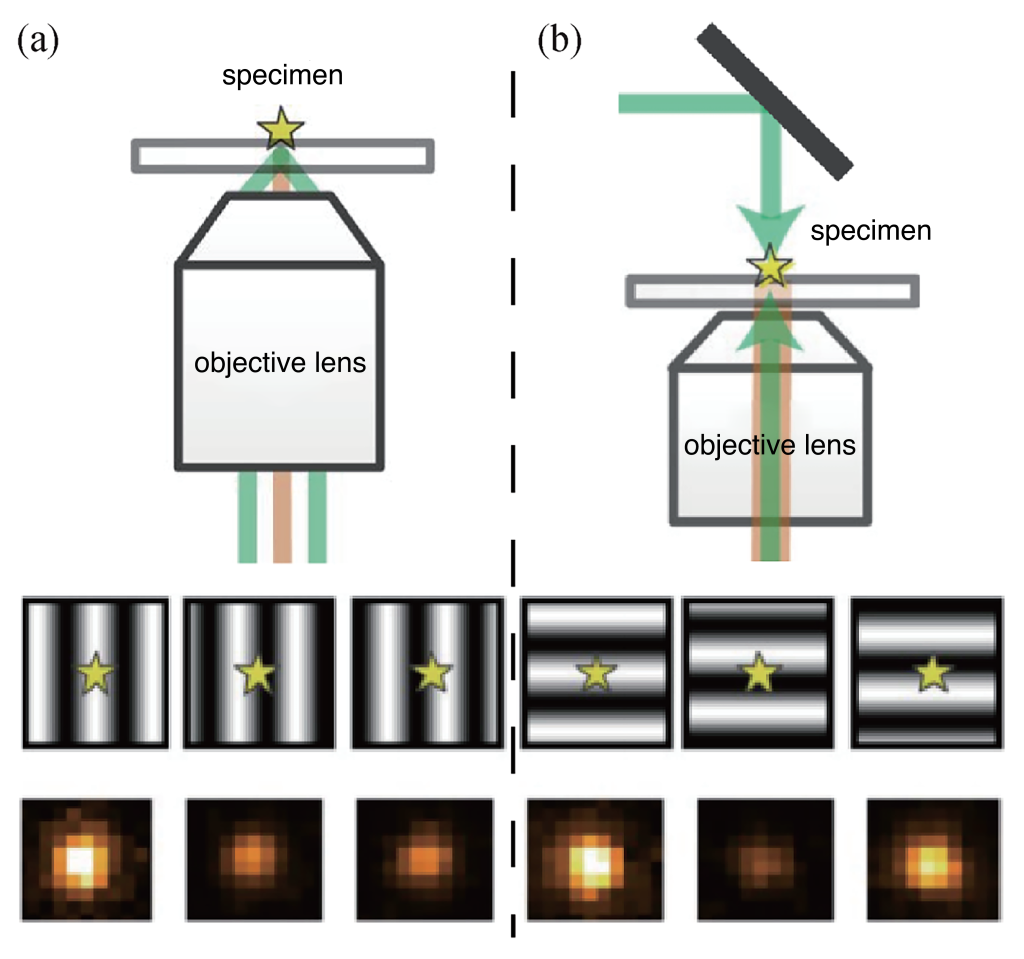
4.2 Axial Interference Single-Molecule Positioning Imaging (ROSE-Z)
For three-dimensional super-resolution imaging, axial resolution improvement is crucial. The ROSE-Z technique, applying interference-based positioning in the axial direction, significantly enhances axial positioning accuracy. This approach has been applied to resolve various subcellular structures, demonstrating unique advantages in characterizing nanoscale structures.
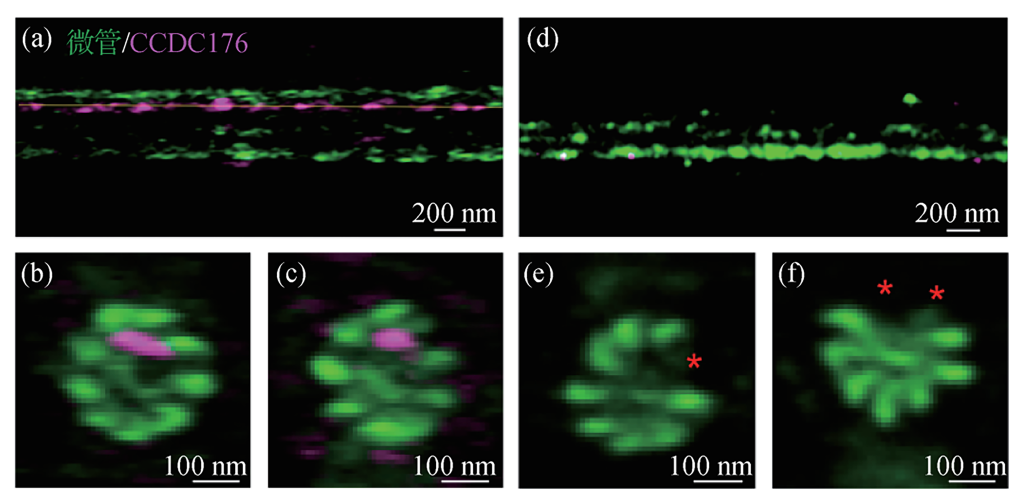
V. Conclusion and Future Perspectives
Super-resolution imaging technologies have surpassed the diffraction limits of traditional optical microscopes, enabling the observation of various intricate structures within cells and the investigation of the spatial distribution of target protein molecules. The continuous development of various super-resolution microscopy techniques, exemplified by single-molecule localization microscopy (SMLM), provides a powerful research tool for unveiling the mysteries of life.
In recent years, the emergence of nanoscale single-molecule localization imaging techniques, distinct from traditional centroid fitting based on single-molecule images, utilizes light fields with different intensity spatial distributions to excite fluorescent molecules. By analyzing the signal intensity of individual fluorescent molecules about the light field, this new approach determines the spatial positions of molecules. The advent of this technology has introduced new means for life science research and has gradually found applications in in-situ imaging studies of large biological molecular machinery. For instance, the Ardem Patapoutian research group recently employed nanoscale resolution imaging technology, combined with site-specific insertion of unnatural amino acids and click chemistry labeling techniques, for in-situ imaging of the mechanically sensitive ion channel protein Piezo 1 on the cell membrane. The study demonstrated the high flexibility of the protein subunit’s C-terminus, a region that electron microscopy struggles to resolve. By imaging the protein’s C-terminus in the presence of small molecule agonists and antagonists, distance information was obtained, elucidating the mechanism behind Piezo 1 protein conformational changes.
Single-molecule localization super-resolution technology boasts the highest spatial resolution in optical imaging. When combined with techniques such as optical slicing and adaptive optics, it can achieve whole-cell super-resolution imaging. Furthermore, the integration of ROSE-XY and ROSE-Z in the ROSE-3D technique holds promise for maintaining the same high level of accuracy in the localization of fluorescent molecules at different depths, enabling isotropic super-resolution imaging of thick samples. With the emergence of more single-molecule imaging and analysis methods, combined with technologies like deep learning, advancements in super-resolution fluorescence microscopy will continue to drive progress in various fields, including life sciences and medicine, by enhancing spatial, temporal, and depth resolutions.