The microscope is the primary tool for observing cells. According to different light sources, they can be divided into two categories: optical microscopes and electron microscopes. The former uses visible light as the light source, and the latter uses electron beams as the light source.
Optical microscopes are mainly divided into the following types: optical microscopes, electron microscopes, phase microscopes, ultrasonic microscopes, and biological microscopes.

Ordinary optical microscopes usually use natural light or light as the light source, with a wavelength of about 0.5μm. Under optimal conditions, the maximum resolution of the microscope is 0.25μm, and the smallest image that can be seen by the naked eye is 0.2mm. Therefore, under an ordinary optical microscope, Using an oil lens to magnify 1000 times, the 0x.25μm particles can be magnified to 0.25mm, which can be seen with the naked eye. Generally, bacteria are larger than 0.25μm, so they can be seen with an ordinary optical microscope.
Ordinary optical microscopes and confocal laser microscopes are both optical microscopes.
Compared with ordinary optical microscopes, laser scanning confocal microscopy has the characteristics of high definition, high resolution, and high sensitivity. It is widely used in the biomedical field and is an important imaging tool in this field.
Confocal laser scanning microscopy
A confocal laser scanning microscope (CLSM for short) is a modern biomedical imaging instrument. It installs a laser scanning device based on fluorescence microscope imaging. It uses ultraviolet light or visible light to excite fluorescent probes and rapidly scans the imaging point by point, line by line, and surface by surface. The scanning laser and fluorescence collection share an objective lens, and the objective lens scans the laser. The focus point is also the object point of instant imaging.
Computers are used for image processing to obtain fluorescence images of the internal microstructure of cells or tissues and to observe physiological signals such as Ca2+, pH value, membrane potential and changes in cell morphology at the subcellular level.
Because the wavelength of the laser beam is shorter and the beam is very thin, the laser confocal scanning microscope has a higher resolution, which is about three times that of an ordinary optical microscope.
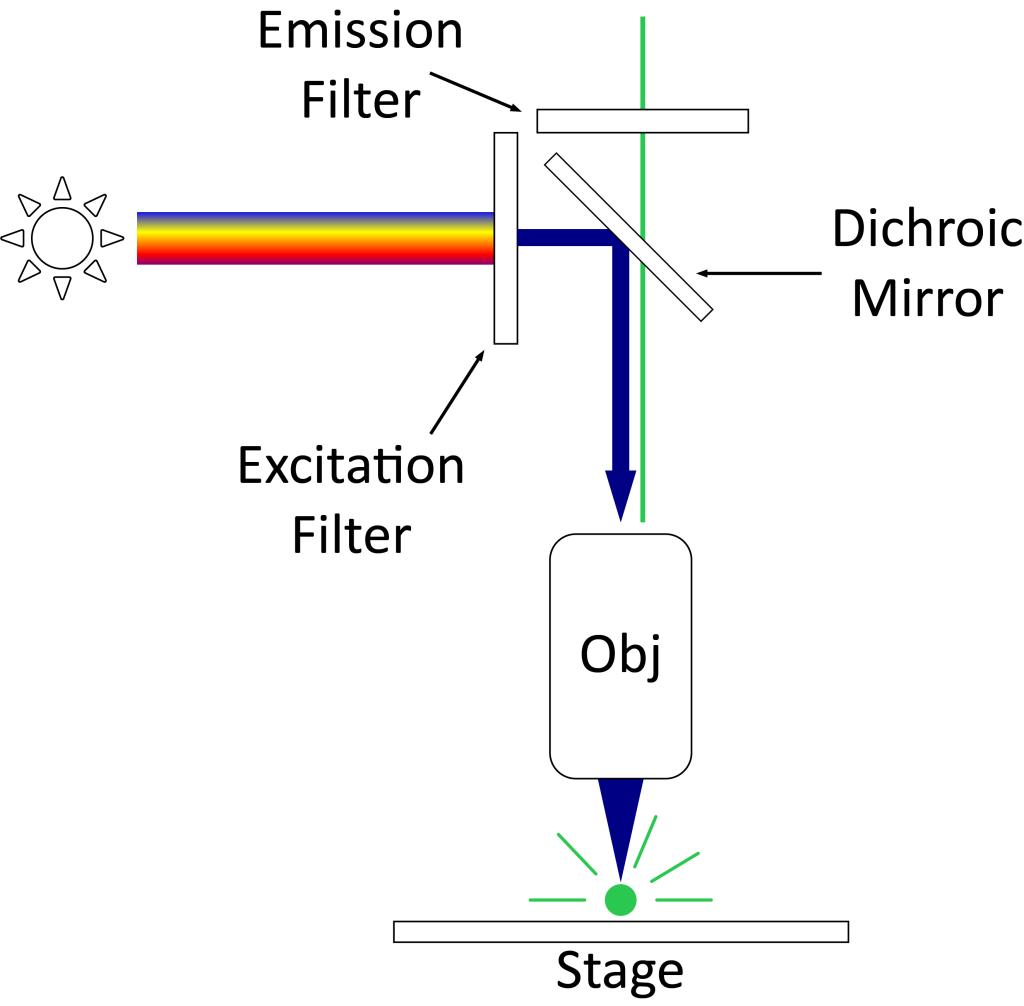
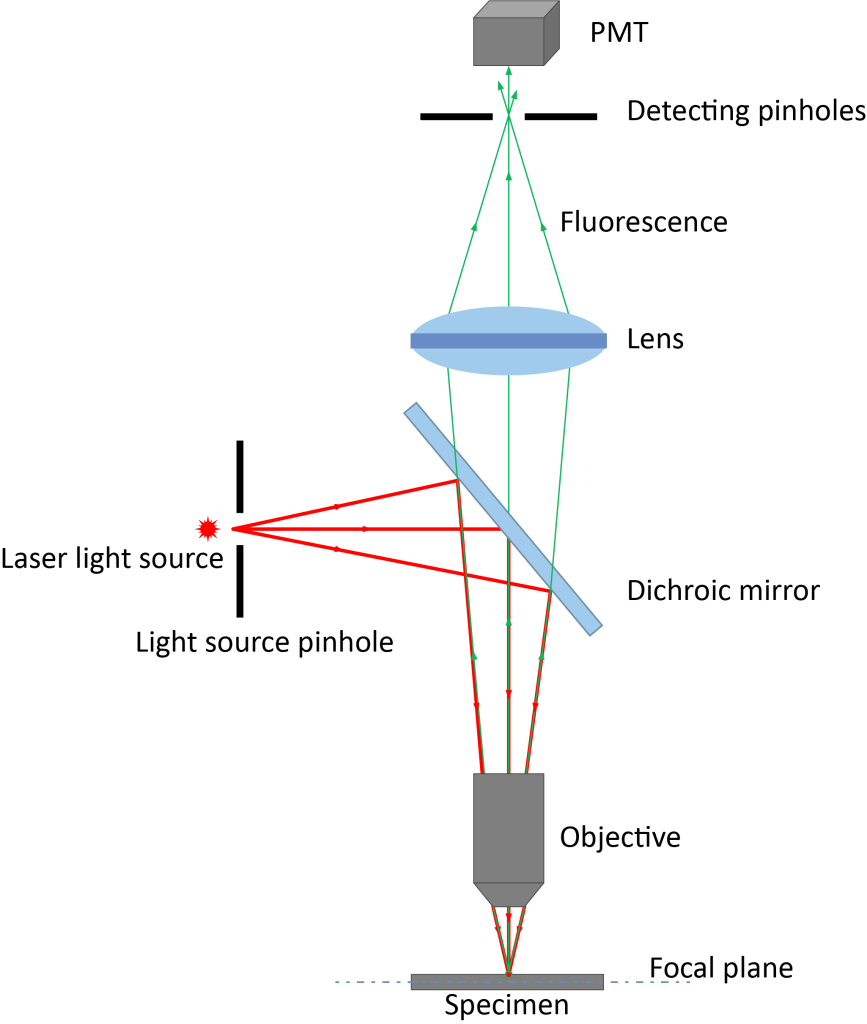
Figure 1 The difference in optical principles between ordinary fluorescence microscope and confocal laser scanning microscope
Confocal microscopy has three advantages:
1. Only collect signals on the focal plane to make the image clear from blur.
The confocal microscope achieves this function mainly because of the key hardware of confocal – the pinhole, which is a small hole in front of the detector and is placed on the optical plane conjugated to the focal plane of the sample. When the excitation light irradiates the sample, the sample area on the focal plane and the non-focal plane will be excited to generate fluorescence signals. Adjust the appropriate pinhole size (usually 1 Au) so that the fluorescence signal on the focal plane can reach through the pinhole. The detector (shown in Figures 2 and 3 is a multi-color image taken on a flat surface), and the fluorescence signal outside the focal plane cannot reach the detector through the pinhole. By adding hardware pinholes, confocal can obtain clear and high-quality images without changing the production method of ordinary fluorescence microscopes.
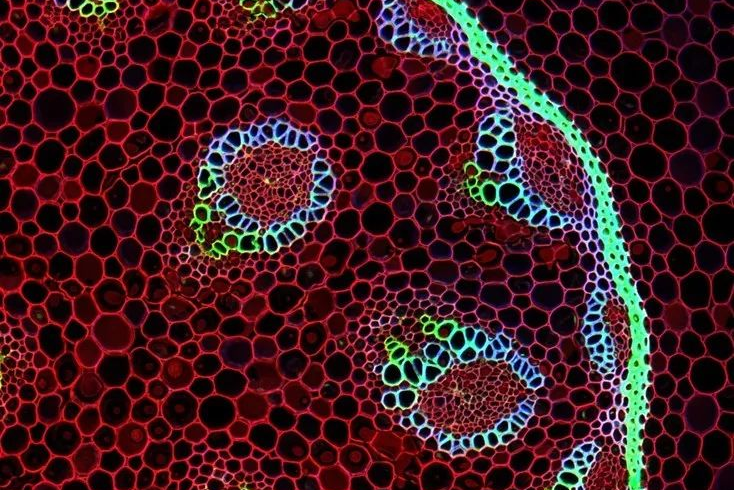
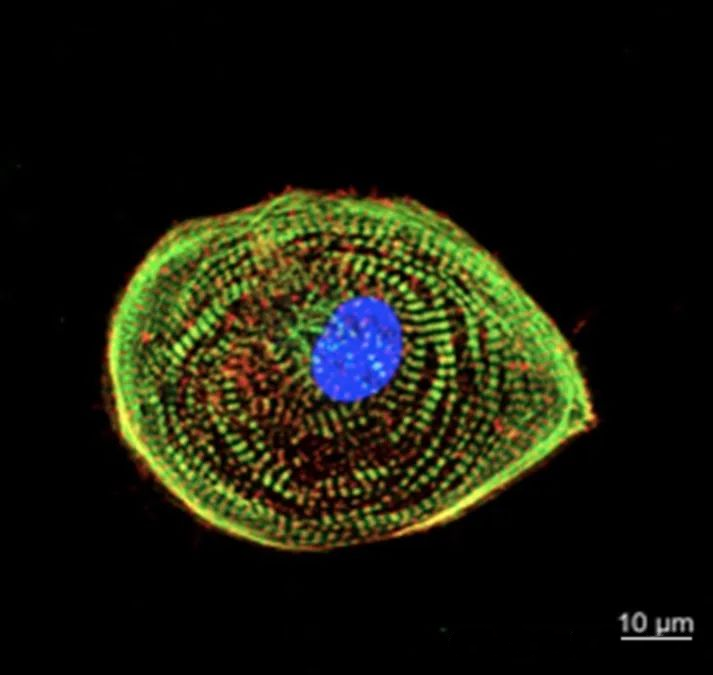
2. Can continuously acquire signals from different focal planes to achieve three-dimensional reconstruction
Due to the existence of pinholes, we can only obtain signals on the focal plane and obtain high-resolution two-dimensional images. By continuously adjusting the Z-axis position, you can obtain two-dimensional images at different Z-axis positions and obtain a series of continuous “optical slice” images. After obtaining these optical sections, the 3D module equipped with the instrument software can be used to reorganize the three-dimensional images to construct a clear 3D image (as shown in the 3D imaging of rhizome sections in Figure 4). This non-destructive, continuous collection of optical slice images realizes the function of “cell CT”.
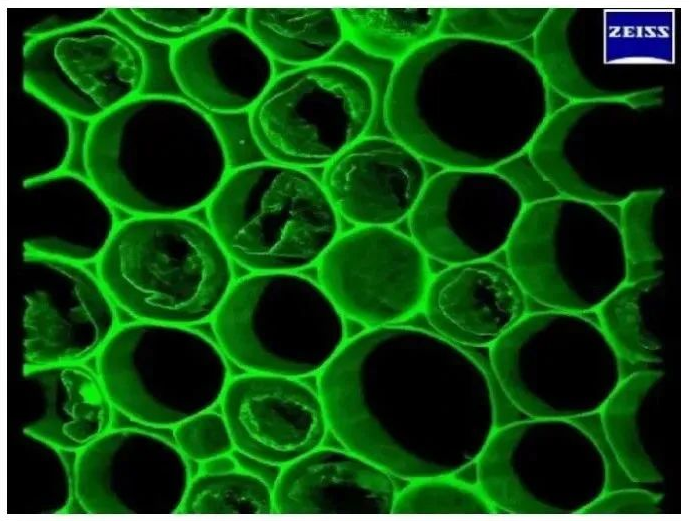
3. Applied to co-localization research
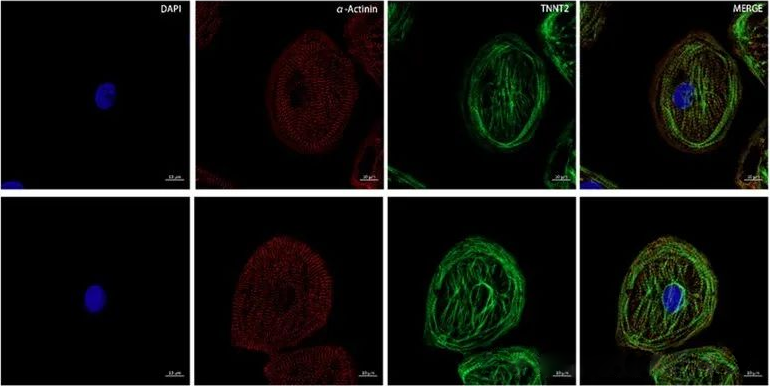
The accuracy of co-localization results is closely related to the resolution of the image, so we need to choose a higher-resolution imaging method when conducting co-localization experiments. Compared with traditional microscopes, confocal microscopy has the advantage of high resolution and has become a powerful tool for colocalization research. Individual nuclear localization can be observed as shown in Figure 5.
Due to the existence of the diffraction limit, conventional confocal optical resolution is around 200nm. When we need to observe structures larger than 200nm, confocal can accurately determine the degree of co-localization.