1. What is Cryo-TEM?
Cryogenic transmission electron microscope (Cryo-TEM) is based on the traditional transmission electron microscope, plus a cryogenic transmission system and a freezing anti-pollution system.
By rapid freezing, the sample is vitrified and takes on a glassy appearance without ice crystals forming. In this state, samples deteriorate much more slowly, allowing for the study of biological materials.
Cryo-electron microscopy combined with three-dimensional reconstruction technology has developed rapidly in the field of structural biology in recent years and has achieved great results in the study of the structure of biological macromolecules, especially the structure of supramolecular systems.
2. Principle and structure of cryo-electron microscope
The core of cryo-electron microscopy analysis of biological macromolecules and cell structures is transmission electron microscopy imaging, and its basic process includes several basic steps such as sample preparation, transmission electron microscopy imaging, image processing and structure analysis.
2.1 Working principle
2.1.1 Sample preparation: sample rapid freezing technology
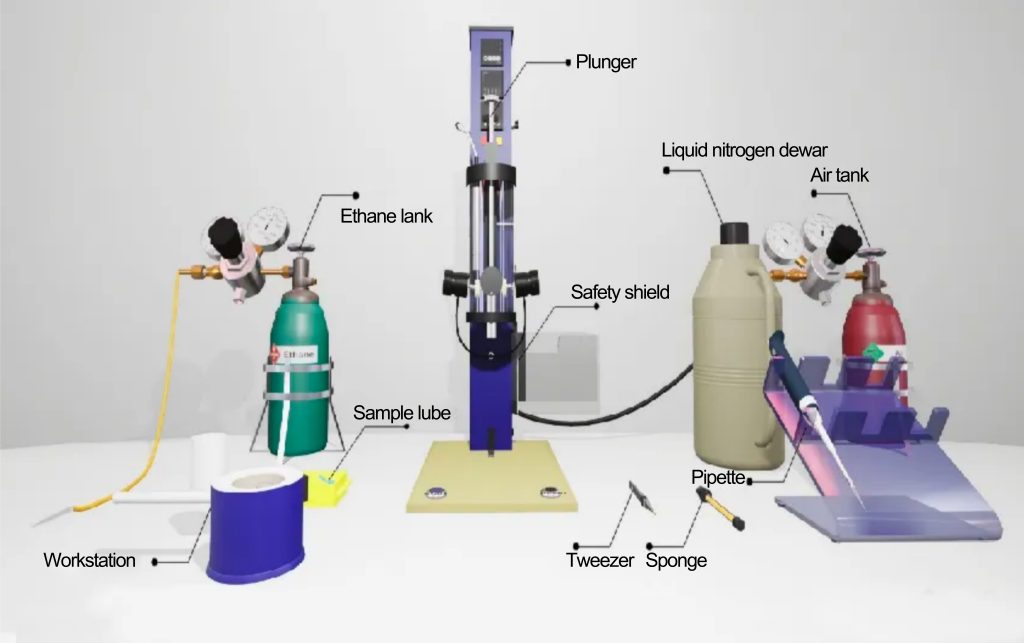
In situ, cryofixation of samples is the first step in cryo-EM specimen preparation, and the process is shown in the figure below [1].
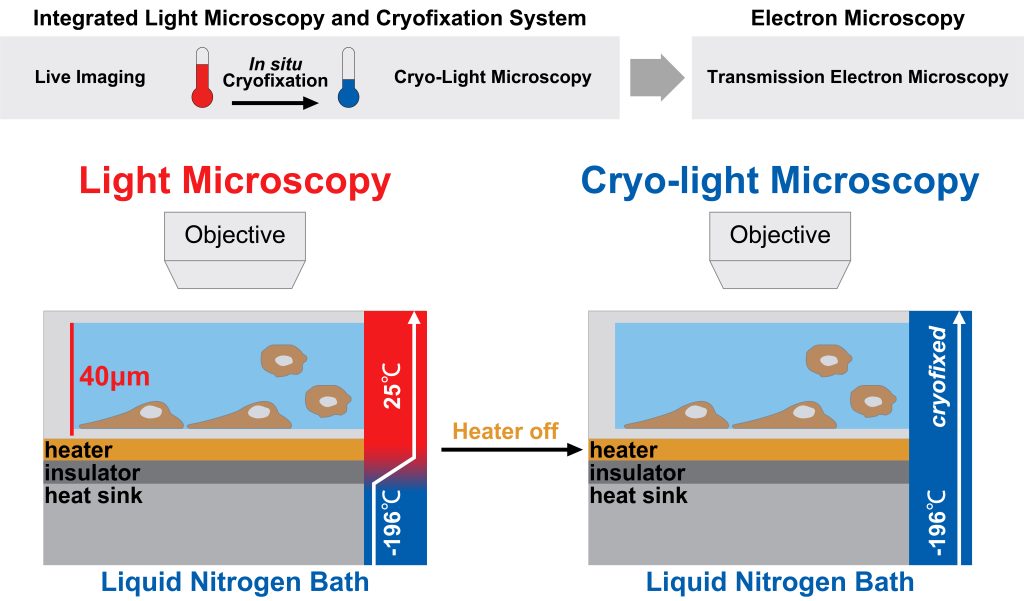
The key to the rapid freezing technology adopted by the cryo-electron microscope is “fast”.
This is because using conventional freezing methods, water molecules will form ice crystals under the action of hydrogen bonds, which will change the sample structure, and secondly, during the imaging process, ice crystals will produce strong electron diffraction to cover up the sample signal.
When the freezing rate is fast enough, the water molecules will solidify into amorphous glassy ice before forming crystals, which have non-crystalline characteristics, which ensures that it will not interfere with the imaging of the sample during the electron beam detection and imaging process.
For freeze fixation, the sample is first placed in a container cooled with liquid nitrogen and then quickly immersed in liquid ethane.
The purpose of using liquid ethane as a cryogen is to make the freezing rate fast enough. During the freezing process, the sample will be rapidly cooled at a speed of 104 to 106 K per second.
After the water in the biological sample is vitrified, the structure of the sample is maintained and fixed, and the vitrified ice will not volatilize in the vacuum environment, which protects the sample from the damage of electron radiation to a certain extent.
2.1.2 Sample imaging: low-dose irradiation imaging
When performing TEM characterization on common sample materials, the higher the electron dose, the better the imaging quality. However, the radiation damage to biological samples is related to the total cumulative radiation dose. In more detail, with the increase of radiation dose, the damage of radiation damage to high-resolution details is more serious. Therefore, in order to obtain as much detail as possible, it is necessary to image the sample with low-dose radiation.
In cryo-electron microscopy, there are two commonly used low-dose radiation imaging methods: cryo-electron tomography and single-particle analysis imaging.
- Electron tomography (cryogenic computed tomography)
During tomography, the sample is continuously rotated and an image is taken at each rotation angle. Each electron microscopic image is a two-dimensional projection image of an object in different projection directions. After Fourier transform, a series of cross-sections with different orientations will be obtained. When there are enough cross-sections, three-dimensional information in Fourier space will be obtained, and then through Fourier inverse transform. The three-dimensional structure of the object can be obtained.
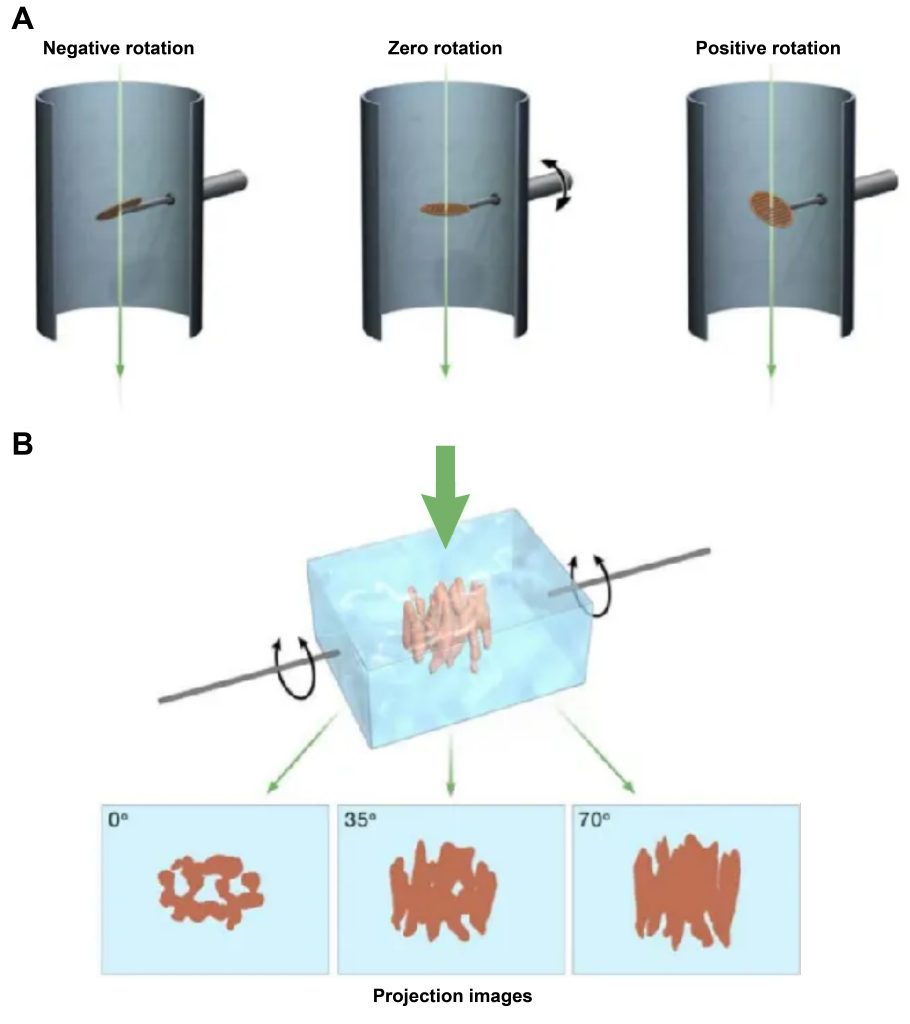
- Single particle analysis (SPA)
The specific method of obtaining projection by single particle technology is shown in Figure 3: Prepare many macromolecular samples with the same structure, disperse and freeze them, and take random projection photos, and then measure the angle through calculation and simulation, and combine particles with the same angle. Highlight the more specific and easily explained features.
Single-particle cryo-electron microscopy is a technique for reconstructing a single particle, but our research objects are often multi-conformation or structurally heterogeneous proteins, and there are subtle differences between particles, which is one of the important reasons why some proteins cannot obtain high-resolution structures one.
For the analysis of structurally heterogeneous samples, we need first to divide the sample into several homogeneous subsets, and then perform 3D reconstruction separately.
Due to the higher theoretical imaging resolution of single-particle analysis, especially when analyzing samples with homogeneous structures, it shows more convenience and better imaging capabilities, so it has been more widely used. The research objects of single particle analysis can be particles with certain symmetry, or protein molecules or complexes without any symmetry, especially for the characterization of ribosomes.
- Comparison of electron tomography and single particle analysis:
Single Particle Analysis:
Advantages: The theoretical resolution of analyzing biomacromolecules can reach the atomic level; the total radiation value of the sample is small; the analytical resolution of symmetrical particles is higher; the larger the molecular weight, the better the result;
Disadvantages: There are very strict requirements on the repeatability of samples, and they can only be used for the three-dimensional reconstruction of purified biomacromolecules with stable structures.
Electron Tomography:
Advantages: simple and direct; low requirements for samples; often used for three-dimensional reconstruction of cells or biological tissue structures;
Disadvantages: When taking multiple photos of the same sample position, the radiation damage of the electron beam to the sample becomes a more serious problem; the rotation angle of the sample is limited by the ability of the electron beam to penetrate the thickness of the sample.
2.1.3 3D reconstruction
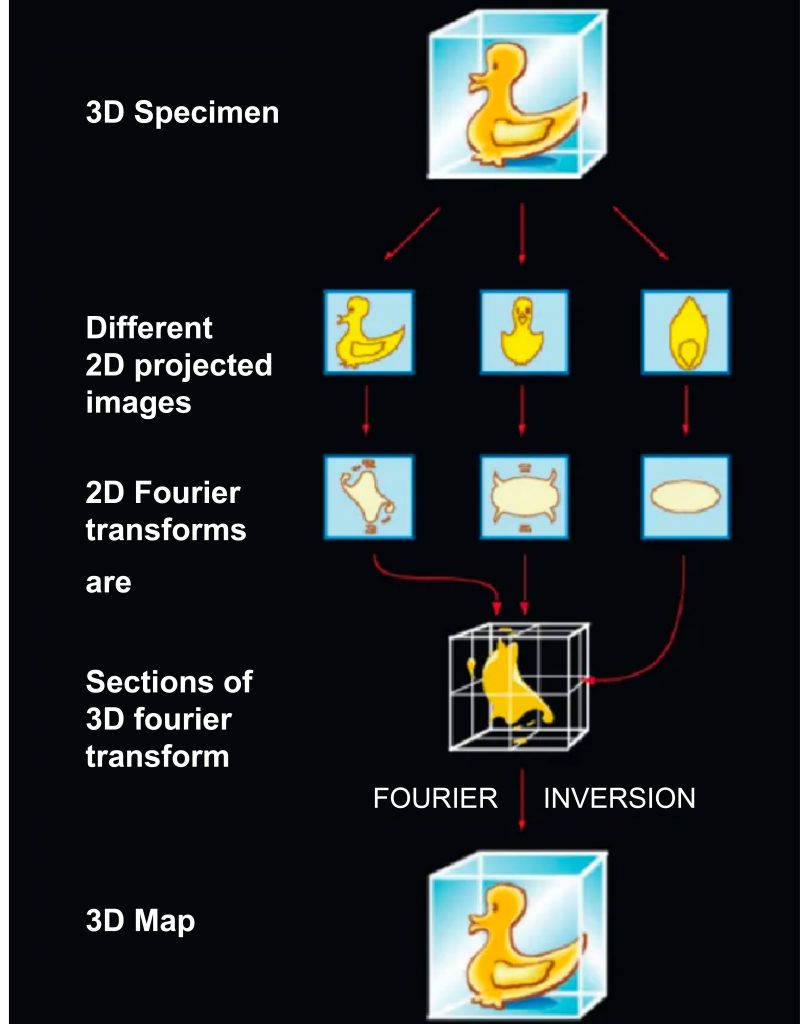
The image formed by the transmission electron microscope is a projected image of the object, similar to an X-ray film. By using the projective slice theorem (Fig. 4), many images of an object taken from a range of viewing angles (2D projections) can be combined to generate a 3D reconstruction of the object.
Under ideal single-particle imaging conditions, proteins in glass ice are randomly distributed, and isotropic reconstructions can be achieved if a large number of particle images are used. This is in contrast to electron tomography, which limits the viewing angle due to the geometry of the sample, enabling anisotropic reconstruction.
2.2 Workflow
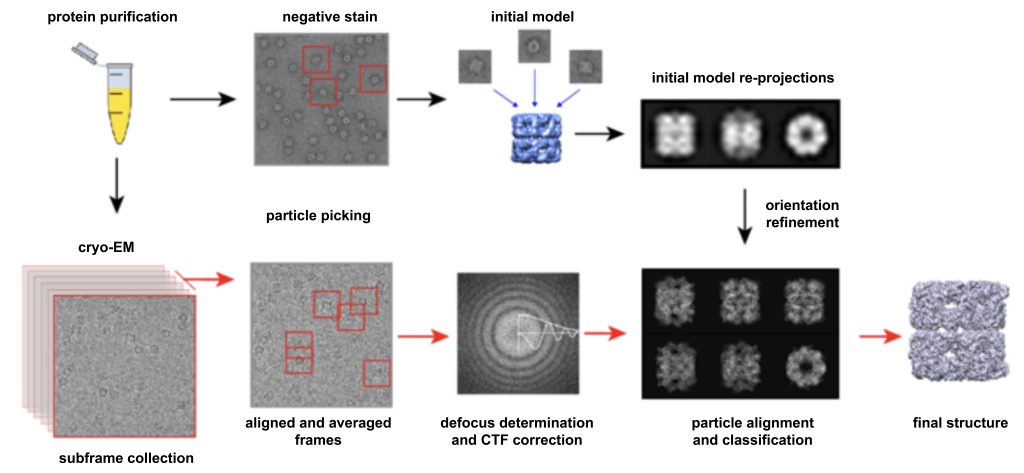
The workflow of single-particle cryo-EM is as follows:
Step 1: Sample preparation. A high-purity, high-concentration protein sample solution is dropped on a particular sample carrier. The carrier net is composed of an ultra-thin amorphous carbon film full of small holes and a metal support frame.
Under surface tension, a thin water film across the pores will be formed on the micropores. After the excess solution is sucked away, the grid carrying the ultra-thin film of the protein solution is quickly put into the liquid ethane cryogen to freeze it quickly, so that the protein is dispersed and fixed in the glassy ice film.
Step 2: SEM image acquisition. Choose samples with the best particle density and glassy ice thickness most likely to yield the best images, set the best parameters (eg under-focus, magnification, electron dose, etc.), record a large number of images of these sample regions, Use manual or semi-automatic program box to take the projection maps formed by those discrete molecules.
Step 3: Build the 3D structure. Because electrons may cause radiation damage to very sensitive samples, single-particle cryo-EM can only use very low electron quantities, and the 2D projection images of this electron microscope have very large background noise. To improve image resolution, researchers first propose an initial 3D model, then sort the captured 2D images of single particles.
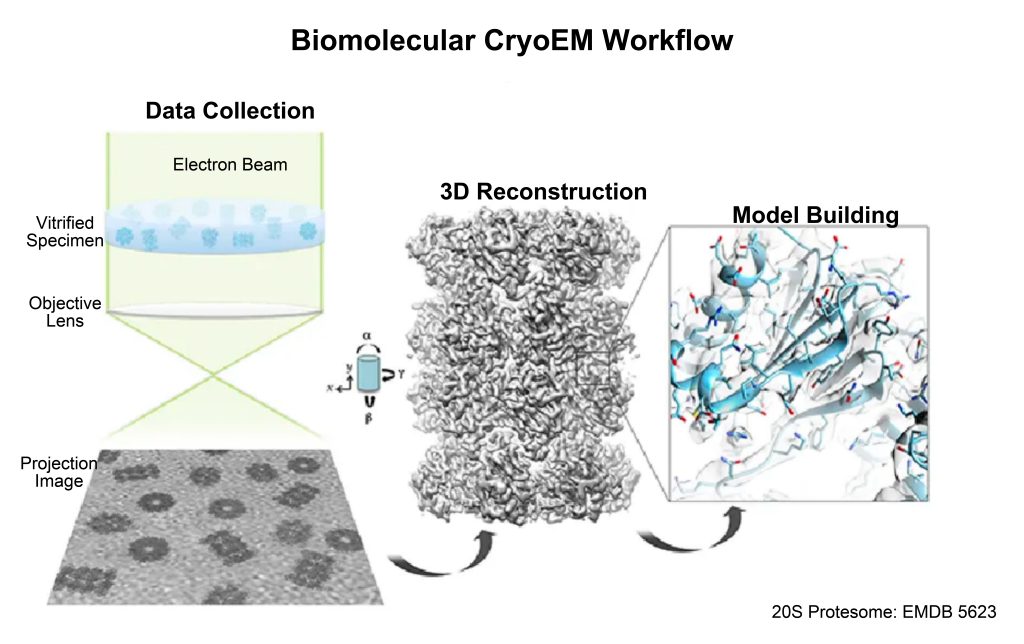
2.3 Instrument structure
The instrument structure of the cryo-electron microscope is similar to that of the transmission electron microscope, except that it is equipped with a liquid ethane tank and a freezer chamber before sample injection to ensure that the sample can be transferred to the sample chamber immediately after rapid freezing.
- Freezing chamber: In actual operation, when a sample is put into liquid ethane, the ethane will boil rapidly around the sample, forming an insulating gaseous film, which slows down the heat transfer to the cryogenic liquid, which is called the Leidenfrost effect answer. It is therefore very difficult to cool water in a sample thicker than a few micrometers at a high enough rate to produce amorphous ice. A rotary vane vacuum pump is added to the freezer to pump the refrigerant in, which can increase the cooling speed.
- Electron gun: the part that generates the electron beam, and the condenser lens system is responsible for focusing the electron beam onto the sample.
- Image generation system: It consists of the objective lens, intermediate and projector lens, and movable platform.
- Image recording system: collect the electronic signal from the sample and form an image on the fluorescent screen.
3. Summary and Outlook
For a long time, cryo-electron microscopy has achieved great success in the field of structural biology. The three-dimensional classification of multi-conformation proteins and the dynamic analysis of biomacromolecules are still challenging research directions. The development of new algorithms will also focus on these issues Expand.
As a low-signal source excitation test technology, cryo-electron microscopy can be used in the physical characterization and mechanism of some materials sensitive to electron beams and heat, such as perovskite materials, some polymer materials, hydrogels, quantum dots, and other fine structures.
There is also great application potential in the research. Stones from other hills can learn. With the improvement of hardware equipment and simulation algorithms, this technology, which leads the research of structural biochemistry into a new era, will surely have a broader application prospect in the future.
[1] Fuest M , Nocera G M , Modena M M , et al. Cryofixation during live﹊maging enables millisecond time orrelated light and electron microscopy[J]. Journal of Microscopy, 2018, 272(2).
[1] Fuest M , Nocera G M , Modena M M , et al. Cryofixation during live﹊maging enables millisecond time orrelated light and electron microscopy[J]. Journal of Microscopy, 2018, 272(2).
[2] Subramaniam S (2006) The SIV surface spike imaged by electron tomography: one leg or three? PLoS Pathog 2, e91
[3] Carroni M , Saibil H R . Cryo electron microscopy to determine the structure of macromolecular complexes[J]. Methods, 2016, 95:78-85.
[4] Li Y, Li Y, Pei A, et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy[J]. Science, 2017, 358: 506-510.
