SEM instruments often also offer X-ray emission spectroscopy with energy dispersive spectroscopy (EDX). Sample atoms that absorb incident electrons can emit an X-ray spectrum that contains element-specific emission lines. Typically, X-rays generated within the top 2 microns of the sample can reach the detector. Individual spectra from specific points or EDX maps representing the spatial distribution of chemical elements can be acquired.
1) Generation of characteristic X-rays:
When the low-energy inner electrons of the sample atoms, such as the K-layer electrons, are bombarded by high-speed incident electrons and escape from their own orbits, the outer high-energy level electrons, such as the L-layer or M-layer electrons, may come to fill.
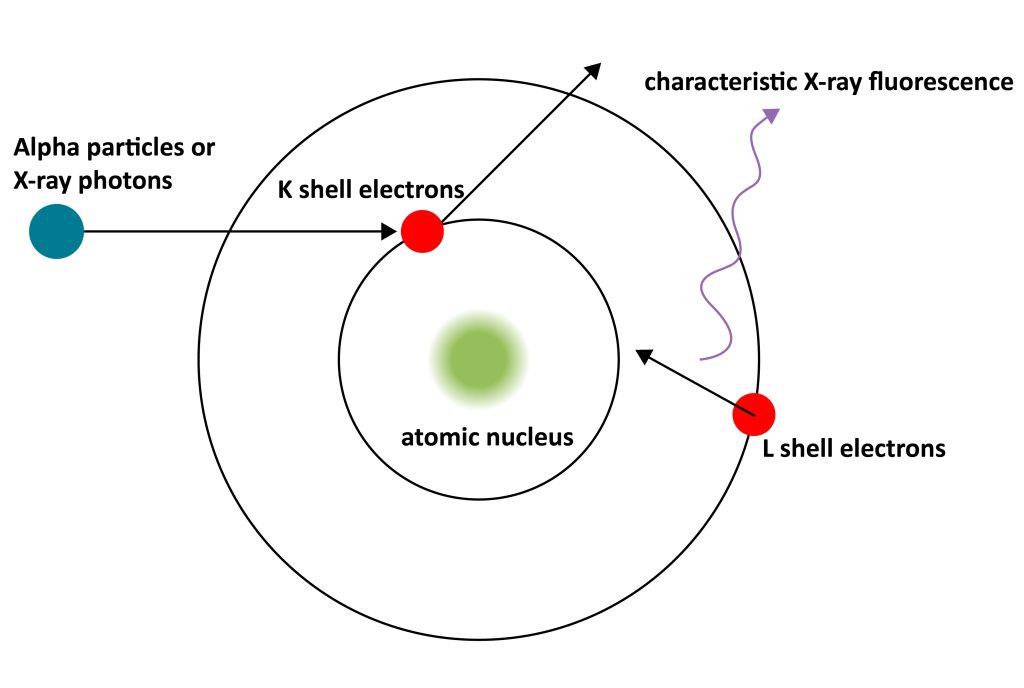
However, electrons in high-energy orbitals need to release excess energy when placed in low-energy orbits. If the excess energy is released in the form of X-rays, an energy level transition occurs and X-rays are emitted.
When the outer electrons of different elements transition to the inner shell vacancies, the X-ray energy released is different, which is the characteristic X-ray, so the element can be determined according to the characteristic X-ray energy.
2) Structural information contained in characteristic X-rays:
The wavelength of X-rays depends on the energy difference between the two energy levels of the inner and outer layers of the element, so the energy or wavelength of the emitted X-rays depends on the element itself. Each element has a fixed position in the X-ray spectrum, so it is called a characteristic X-ray.
The wavelength of characteristic X-rays does not differ due to the energy of incident electrons, but is completely determined by the type of element or atomic number, and is used for composition analysis of substances. Its intensity is proportional to the concentration of related elements, which makes it possible to use characteristic X-rays for quantitative analysis of constituent elements.
The qualitative and quantitative analysis of electron probes and scanning electron microscopes using WDS or EDS is to use the characteristic X-rays generated by electron beam bombardment of the sample.
Each element has a characteristic X-ray wavelength corresponding to it. Different elements are analyzed using different line systems. Light elements use Kα line systems, middle atomic number elements use Kα or Lα line systems, and some heavy elements often use Mα line systems.
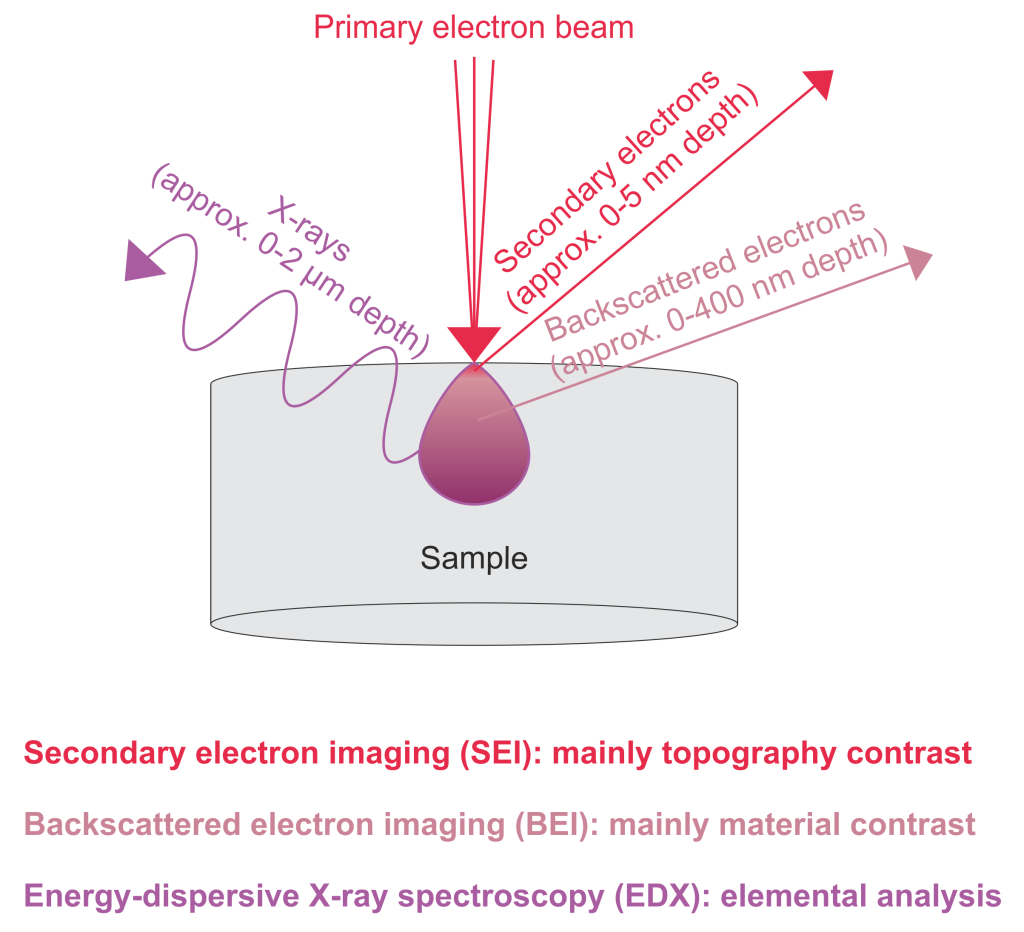
| Signal | Generation Mechanism | Characteristics |
| SE | The incident electron collides with the outer electron inelastically, the energy is lower than 50eV | Reflect the information of the range of the incident electron beam spot, shallow layer; low energy, sensitive to charge, can reflect the difference in valence state and conductivity |
| Ordinary BSE | The incident electrons collide with the sample atoms many times, there are many inelastic collisions, and the energy is higher than 50eV | Produced to a deeper depth, it can qualitatively reflect the difference in element ordinal numbers |
| transmission electron | Electrons passing through a thin sample can be closer to the incident electrons | Only thinner samples are permeable to electrons, with less expansion for high-resolution signals |
| Characteristic X-tay | The incident electron interacts with the inner electron, and the second-ray photon is emitted after the transition of the inner electron | It belongs to electromagnetic radiation, accurately corresponds to element information, and has a significant signal escape depth |
