Three-dimensional imaging is a commonly used technology in life sciences, chemistry, materials and other research. However, conventional three-dimensional imaging technology requires layer-by-layer scanning of the research sample and then uses a computer to synthesize three-dimensional graphics to understand the object structure. When scanning layer by layer, the object to be scanned must remain still during the entire process. Therefore, conventional three-dimensional imaging technology has certain limitations when studying fast dynamic processes.
In order to solve this problem, digital holographic microscopy imaging technology came into being. This technology does not require special markers. It uses the interference phenomenon of light to directly extract the three-dimensional spatio-temporal information of the research object through two-dimensional images. It is a label-free, non-scanning three-dimensional imaging technology that can detect the rapid movement process and three-dimensional topography of organisms. Measurement research shows broad application prospects.
1. Holography and digital holographic microscope
Holography is a technology that records light wave information of an object to reproduce its three-dimensional image.
In 1947, Dennis Gabor, a Hungarian-born physicist at Imperial College London, proposed the concept of holography and recorded the first hologram using chemical film, for which he won the 1971 Nobel Prize in Physics.
Since then, holography has developed a series of technologies such as holographic photography, holographic projection, holographic storage and holographic anti-counterfeiting, and has been widely used in technology, entertainment, industry and other fields.
In the field of microscopic imaging, a new type of microscopic imaging method-digital holographic microscopy has been developed by combining holography, Fourier optics, and digital image processing technologies, which can be used on the basis of conventional optical microscopes. Achieve non-destructive, label-free, rapid and quantitative 3D imaging detection.
Digital holographic microscopy (DHM) is an optical measurement technology that uses the interference phenomenon of light to extract three-dimensional information of samples.
This technology introduces interference on the basis of ordinary optical microscopy imaging, thereby retaining the phase information reflecting the height that is lost during plane imaging in the interference fringes, which is recorded by the camera, and simulates the movement of light in space through subsequent calculations. Propagate and reconstruct the light field in a specific three-dimensional space including the target, thereby achieving quasi-three-dimensional imaging of the sample surface.
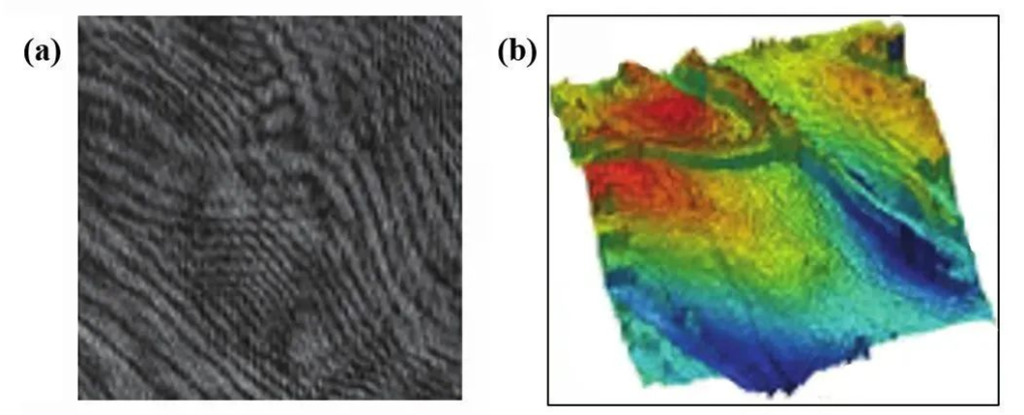
Figure 1. (a) Digital holographic microscopy image (b) Reconstructed three-dimensional phase image of the sample surface
According to different angles, digital holographic microscopes have different classification methods. They have different forms, but the basic principles are the same.
- According to the hologram recording method, it can be divided into coaxial holographic microscope and off-axis holographic microscope;
- According to the way of observing the sample, it can be divided into transmission holographic microscope and reflection holographic microscope;
- According to the path classification of coherent light, it can be divided into the spectroscopic digital holographic microscope and digital holographic microscope with a common object-parameter path.
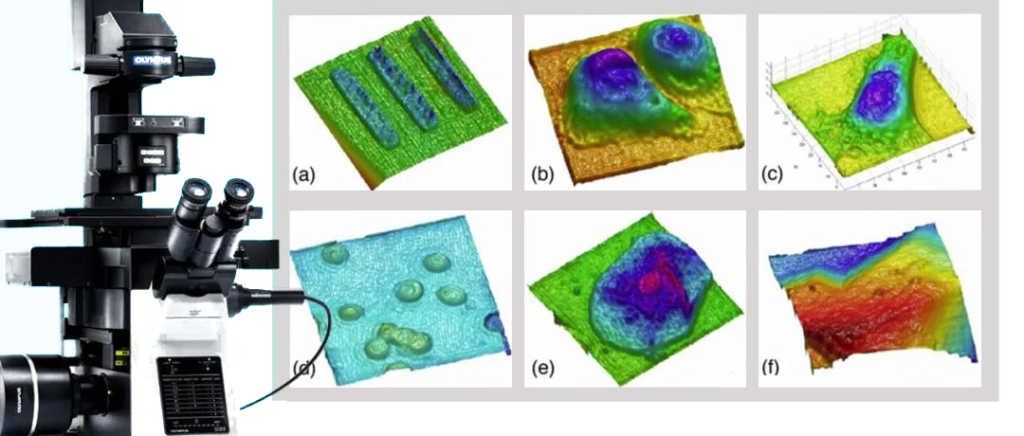
Compared with traditional optical microscopy imaging, digital holographic microscopy imaging can obtain phase information of the sample through calculation. This allows it to be used in the biomedical field, such as realizing real-time three-dimensional morphological observation of cells; in the field of surface detection, digital holographic microscopes can observe the fine three-dimensional morphology of the sample surface, thereby quantitatively dynamically measuring and measuring surface defects. analyze.
2. Coaxial digital holographic microscope
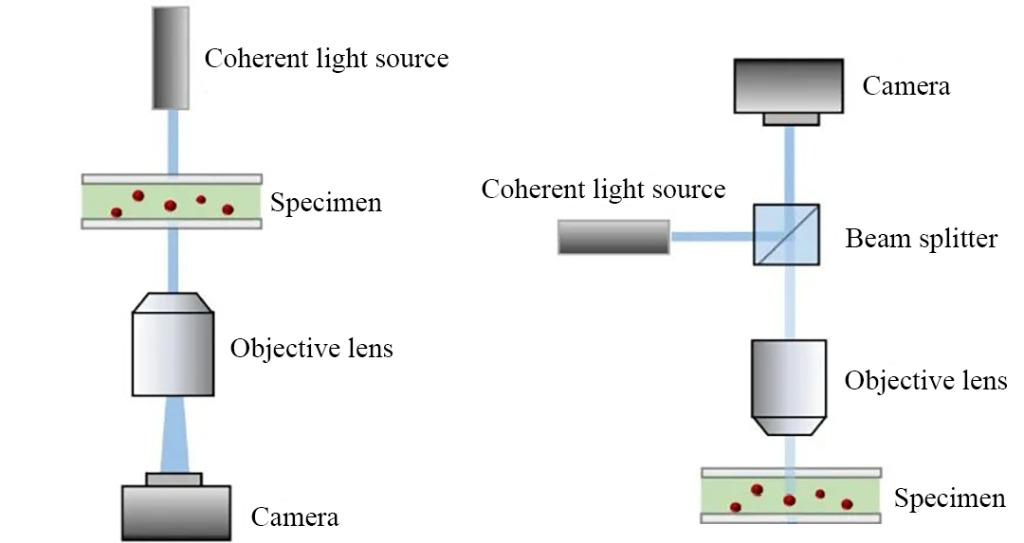
To record a basic hologram, the object light carrying sample information and the reference light used to generate interference need to be illuminated on the recording plane at the same time. Coaxial digital holography is to obtain a hologram when the directions of the reference light and the object light are basically the same.
In order to ensure coherence, the reference light and the object light come from the same light source. The simplest way is to use a low-coherence light source, such as an LED light source that shines on the observation sample. The light is scattered through the sample, and the scattered light interferes with the surrounding blank area to form Ring-shaped interference fringes (Figure 4).
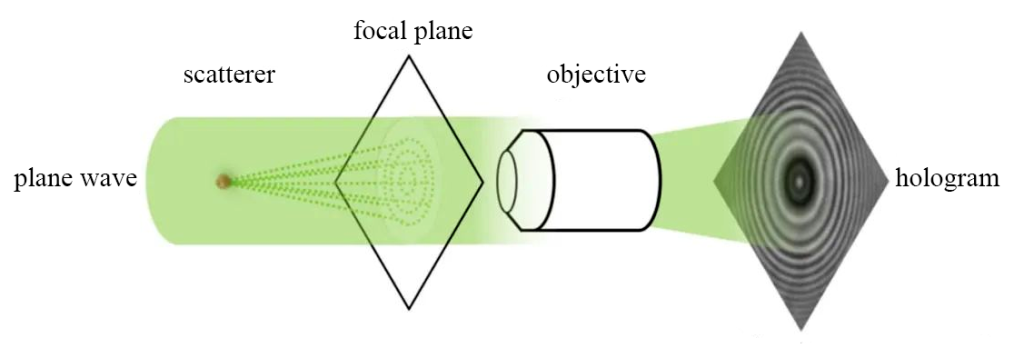
This method has a simple structure and good stability, and can be easily modified from an ordinary optical microscope. Based on the Olympus inverted microscope (Olympus, IX83), a collimated cage LED light path is added to the transmitted light source to build a digital holographic microscope that can achieve 3D real-time detection (Figure 5).
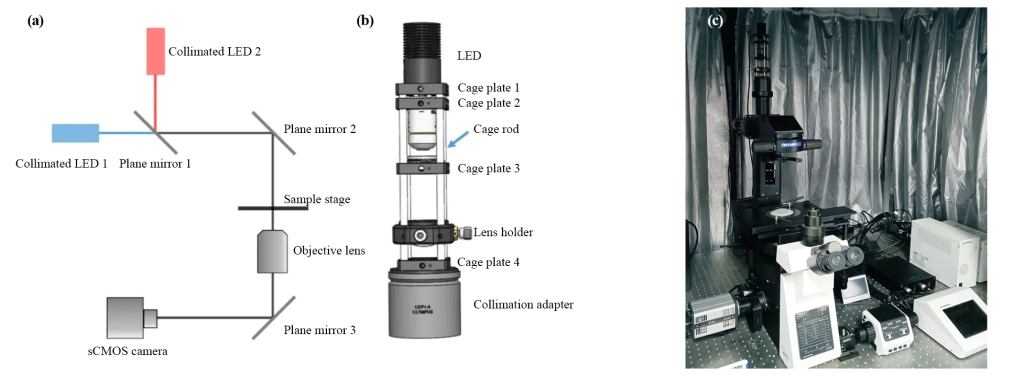
It can also greatly reduce the impact of coherent noise such as subsequent speckle noise and parasitic interference fringes on the hologram, improving imaging quality.
Digital holography does not require staining or other sample preparation processes that affect biological activity during observation, so it is particularly suitable for studying the movement behavior of microorganisms such as bacteria in solutions and near interfaces.
A coaxial digital holographic microscope was used to track and observe the movement trajectory of E. coli near the degradation surface, and then analyze the impact of this interface on bacterial adhesion and other behaviors and the antifouling effect.
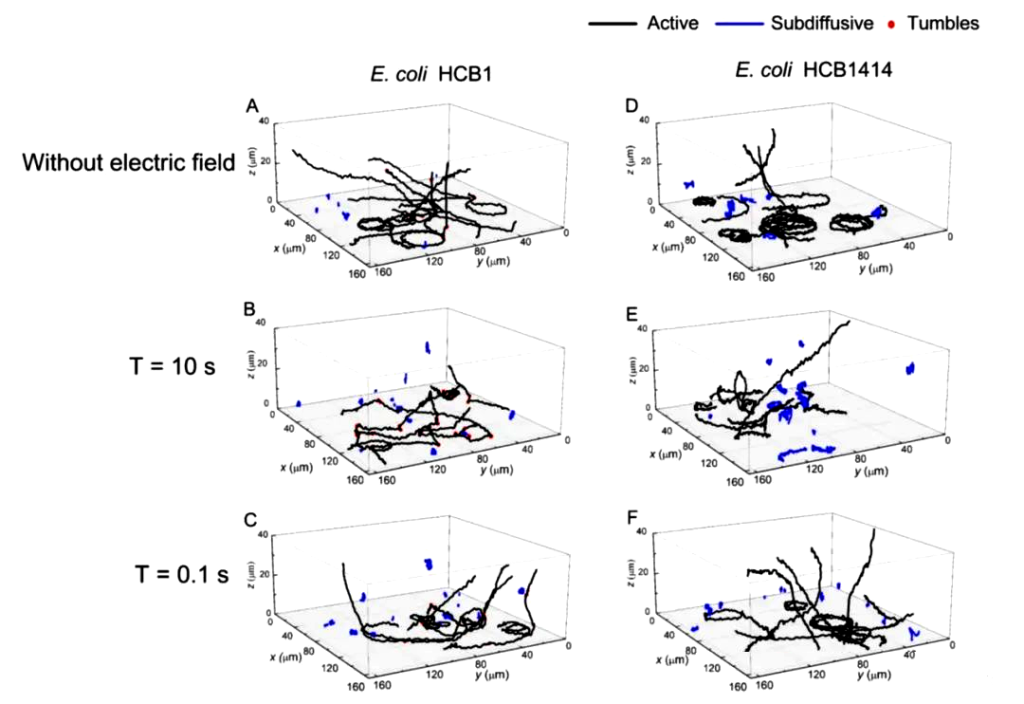
On this basis, a digital holographic microscope was also used to monitor the three-dimensional movement of E. coli near the energized surface under the action of an alternating current (AC) electric field (Figure 6).
By comparing the spatial trajectories under AC electric fields with different periods, it can be found that the adhesion behavior of E. coli is closely related to the period of the AC electric field, which provides inspiration for the use of electric fields for wastewater treatment and marine anti-pollution.
At the same time, the impact of factors such as incident light power, illumination uniformity and pixel size on DHM axial tracking resolution was also studied, and a three-dimensional positioning algorithm based on local maximum intensity search and Gaussian fitting was introduced in the calculation, thereby effectively improving Axial resolution of particles.
Within an observation range of tens of microns deep, the algorithm can achieve an axial positioning accuracy of 58nm for 0.2μm polystyrene particles.
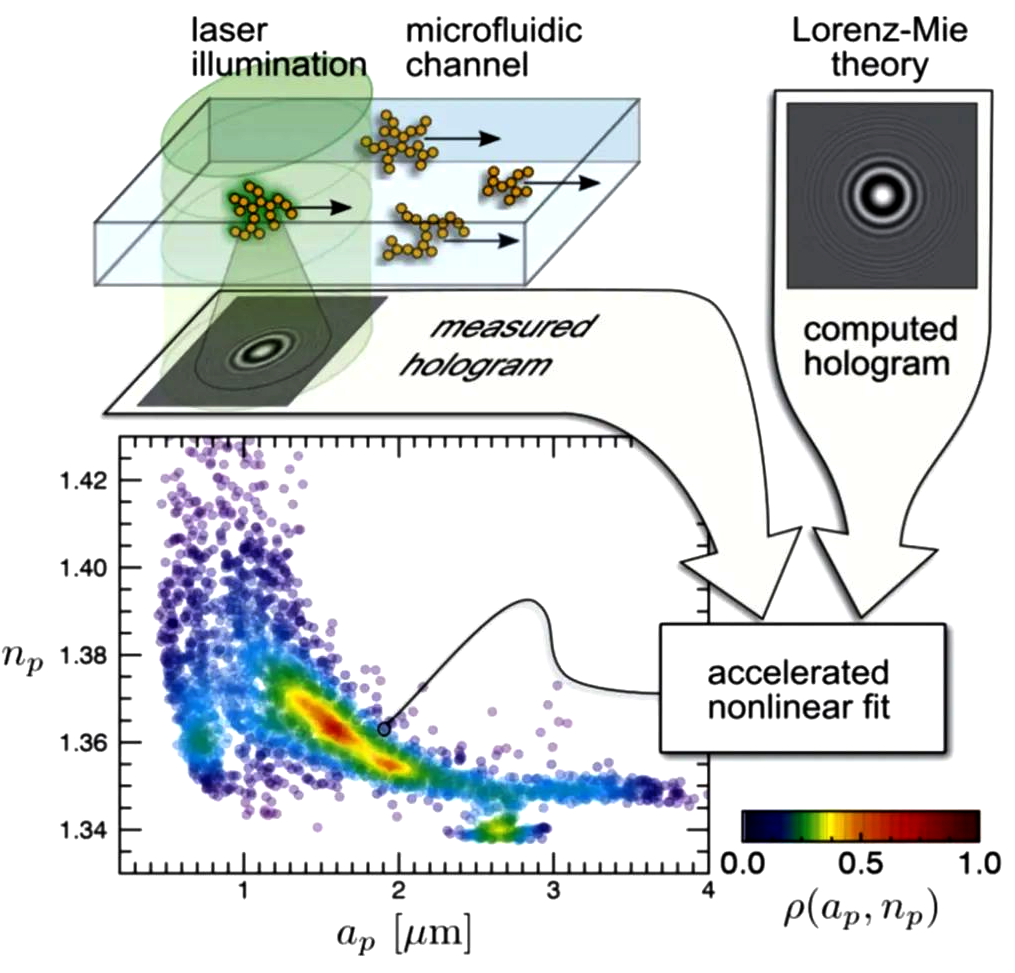
In addition, because the coaxial digital holographic mirror analyzes the interference ring generated by scattering rather than the sample particles themselves, the coaxial digital holographic mirror can break through the diffraction limit of traditional optical systems and observe the movement of extremely small particles.
As shown in Figure 7, Wang et al. from New York University used a coaxial digital holography device to observe bovine insulin aggregates in colloids flowing along microfluidic channels, and combined with the Lorenz-Mie light scattering theory to observe bovine insulin aggregates in colloids. The three-dimensional spatial position, radius ap and refractive index np of the insulin aggregate were statistically analyzed.
However, the above applications cannot observe the overall morphology of the sample surface. Therefore, some researchers have proposed phase-shifted digital holography and off-axis digital holography respectively based on on-axis holography to achieve the characterization of three-dimensional topography.
3. Phase-shift digital holographic microscope
In order to suppress the zero-order and diffractive conjugate images in holograms, Crane first clearly proposed the concept of phase-shift interferometry in 1969. Phase-shift digital holography records multiple phase-shift interference patterns on the same sample by changing the optical path difference between coherent beams, and then calculates the overall phase.
In 1998, some researchers proposed phase-shifted digital holography, using a coaxial digital microscope to obtain multiple holographic images and calculate the size and positional relationship of pollen grains. In 2004, Professor G. Popescu’s research group in the United States used coaxial digital holographic microscopy to measure the division of HeLa cells and the three-dimensional morphology of human red blood cells. The axial measurement accuracy reached 26 nm.

Since multiple holograms need to be recorded, phase-shifted digital holography requires trade-offs in time or space. The time phase shift method records multiple phase shift interference patterns in time sequence. It has a simple structure and is easy to implement, but it is only suitable for stationary or quasi-stationary samples.
The spatial phase shift method can measure the dynamic change process by simultaneously recording multiple phase shift interference patterns through multiple CCD cameras or using different areas of the same CCD. However, the structure is complex, the cost is higher, and there are also problems such as image alignment.
The phase-shifting digital holographic mirror can obtain the overall phase map of the sample and achieve maximum utilization of the camera bandwidth. However, due to the need for phase shifting, highly accurate phase shifters are required, such as piezoelectric ceramics (Piezoelectric Transducer, referred to as PZT), and polarization Phase shifters and gratings are expensive and inevitably have uncertain factors such as phase shift errors and environmental vibrations, which are greatly restricted in their range of use.
4. Off-axis digital holographic microscope
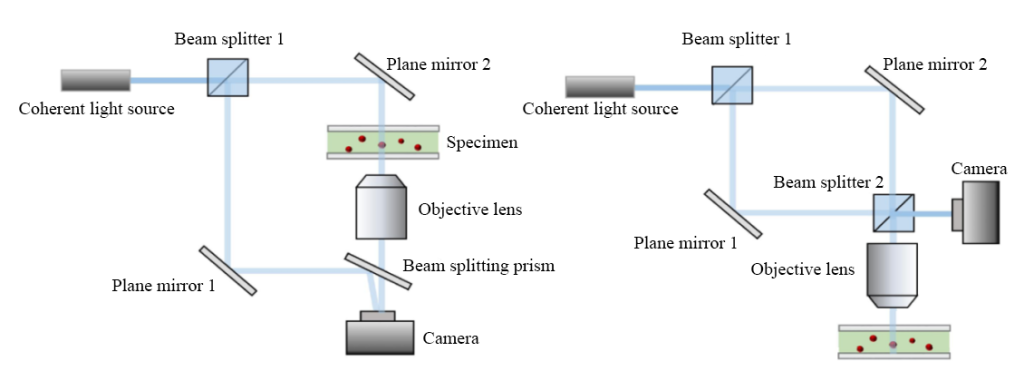
In 1962, American scientists Leith and Upatnieks innovatively proposed off-axis holography, which extended the concept of carrier frequency in communication theory to the airspace and achieved the separation between real images and conjugated virtual images.
The off-axis holography method solves the “twin image” problem in the original on-axis holography method by introducing a limited tilt angle in the direction of reference light propagation and greatly improves the accuracy of the holographic image. Off-axis digital holograms obtain real images through spectral filtering, which inevitably sacrifices a certain bandwidth, but its advantage of restoring the phase of a single hologram allows for convenient dynamic observation.
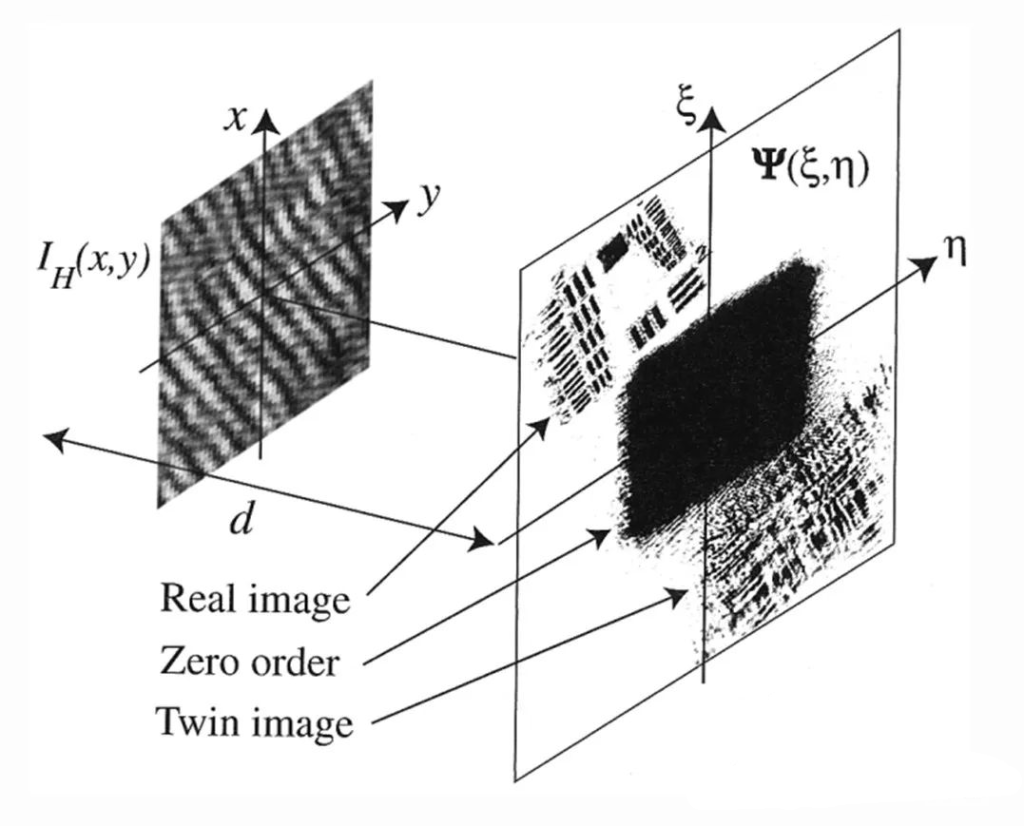
d is the reconstruction distance; Ψ(δ, η) is the reconstructed wavefront.
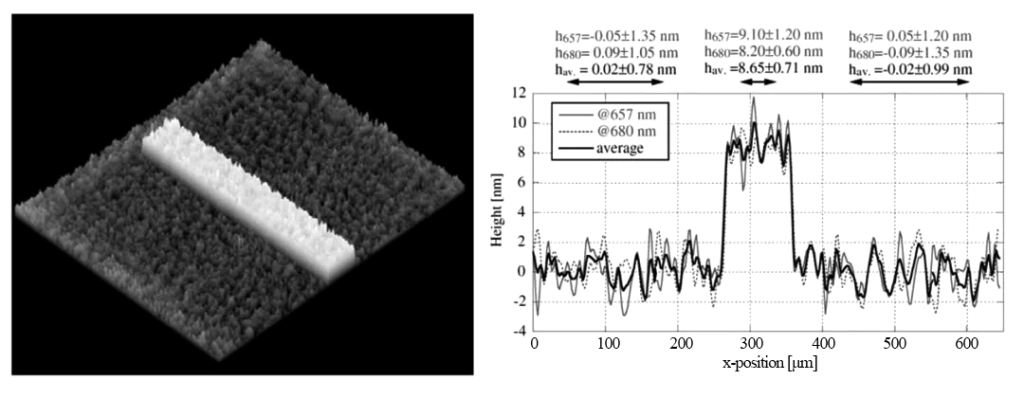
In 2008, the Swiss C. Depeursinge research group used digital holographic microscopy devices at two wavelengths (657 and 680 nm) to conduct three-dimensional measurements of the microstep surface morphology (about 8.9 nm high) evaporated with chromium on a quartz substrate. Analysis, achieving high-precision three-dimensional topography observation.
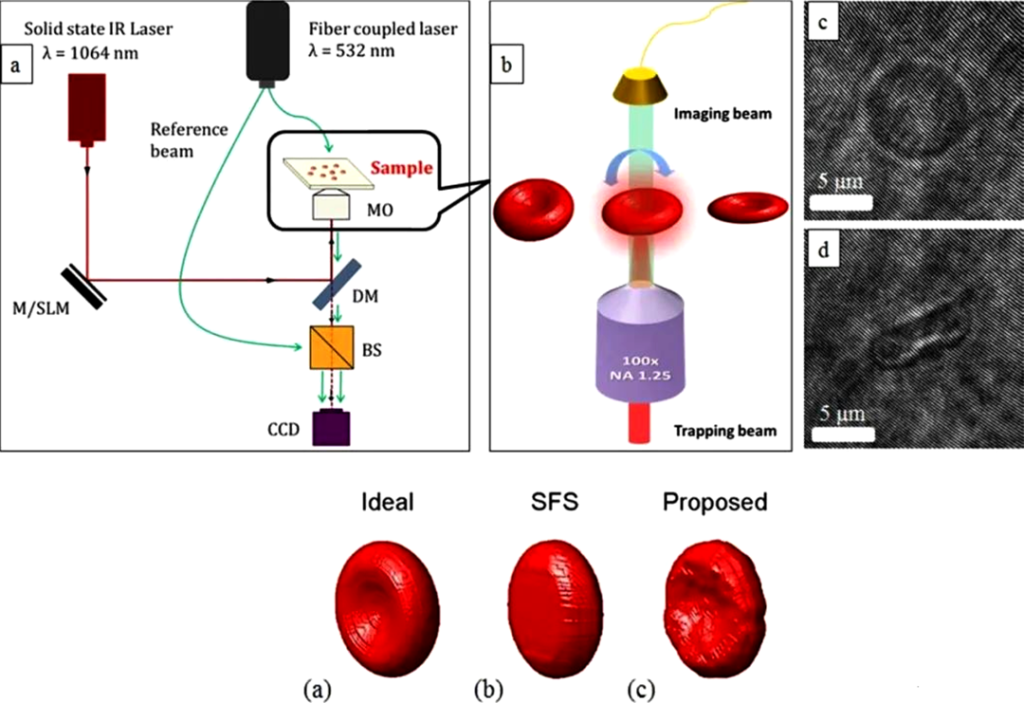
Since the off-axis digital holographic mirror obtains the three-dimensional topography of the surface based on the optical path difference, it can only obtain the three-dimensional topography in one direction for micro-nano particles in space, such as suspended cells, and is insensitive to concave surfaces. To this end, the researchers proposed a method to obtain an overall three-dimensional model of the sample particles by imaging them in all directions. In 2014, Memmolo et al. finally obtained a 3D model of red blood cells by controlling the flipping of red blood cells and recording holograms of red blood cells at different tilt angles. They calculated the decoupling program of the red blood cell reconstruction results for each image.
5. Development and application of digital holographic microscope
In recent years, cheap lasers and cameras with smaller pixel units have provided hardware conditions for further improving the accuracy of digital holographic microscopes. The significant increase in computer computing power has made it possible for digital holographic microscopes to output real-time dynamic results.
In research, multi-wavelength digital holographic microscopy has emerged by changing the optical path structure and using dual-wavelength and three-wavelength light sources to further increase the measurement depth;
Proposing new holographic reconstruction algorithms and image noise reduction algorithms, and verifying them through various simulation calculations, has also become a research hotspot for improving the quality of digital holographic imaging;
Research on improving reconstruction speed and accuracy through deep learning of neural networks and further optimizing imaging has also appeared in people’s field of vision.
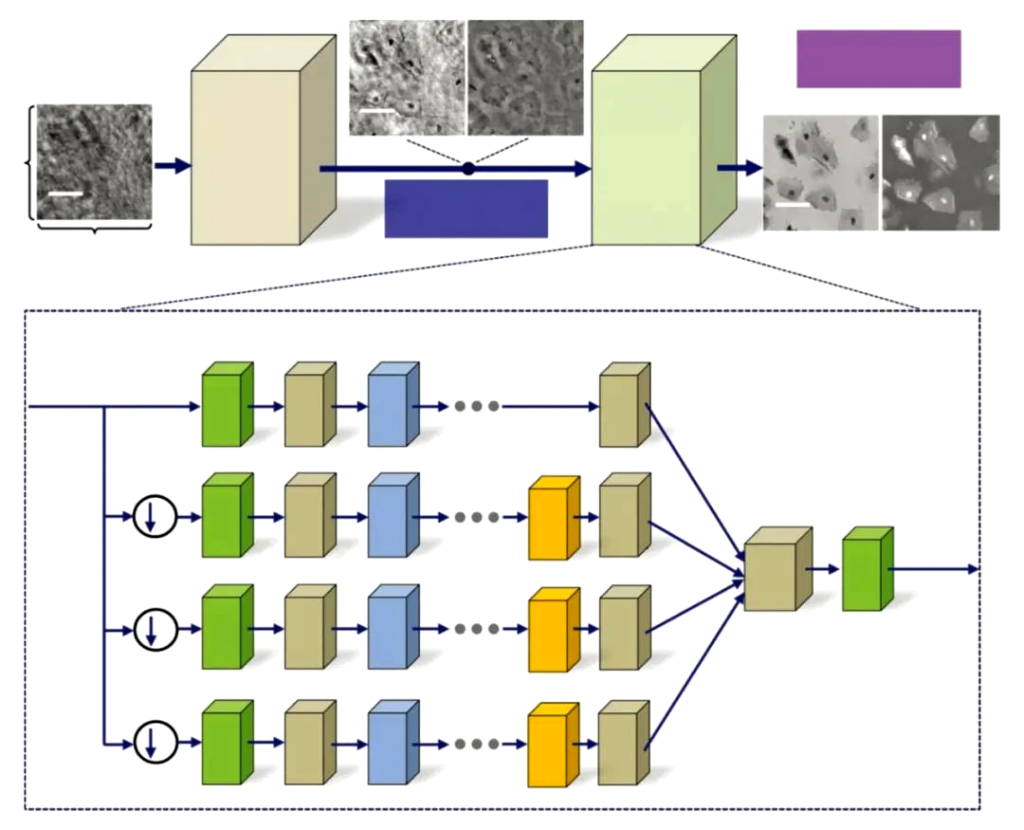
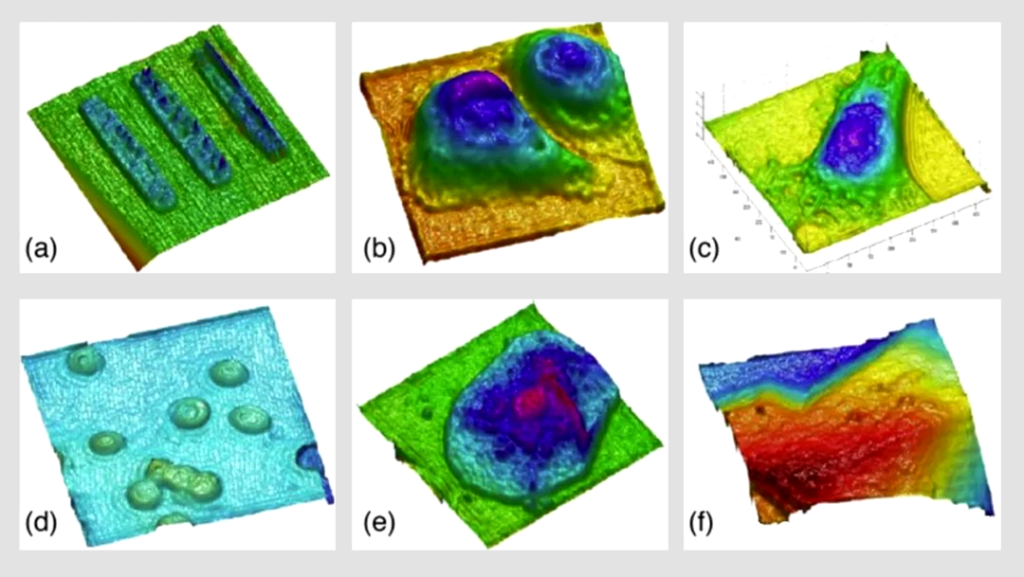
On the other hand, digital holographic microscopes are used in biological cell detection and monitoring (as shown in Figure 13), micro-optical testing, MEMS morphology and deformation measurement, flow field and particle field measurement, vibration analysis and other fields, and its potential is being gradually explored. .
6. Summary and Outlook
Rapid and accurate microscopic three-dimensional imaging is an important issue in basic scientific research. However, traditional methods such as confocal microscopy and atomic force microscopy have limitations such as the need for dyeing and labeling, and are time-consuming.
As a non-contact, label-free, and damage-free rapid quantitative three-dimensional imaging method, digital holographic microscopy enables us to achieve label-free, in-situ, real-time three-dimensional observation of micro-scale particles without introducing external interference. Obtain the changes in its spatial three-dimensional information over time.
However, due to limitations in camera size and resolution and the influence of factors such as laser speckle, digital holographic microscopes make it difficult to obtain high-frequency information from samples, cannot conduct large-area observations, and have difficulties in color restoration.
At present, digital holographic microscopes are becoming increasingly mature. With further research on computer image processing and deep learning algorithms, as well as improvements in laser modulation technology and breakthroughs in the accuracy and acquisition speed of scientific cameras, in the near future, this technology will It will be used in more micro-field research and even in the production and testing of high-precision devices, blooming its unique brilliance.