In 1928, Indian scientist C.V. Raman observed a phenomenon: when light passes through a transparent medium, the frequency of part of the scattered light changes. This phenomenon is called Raman scattering. It was also observed in the Soviet Union and France later that year.
§1 What is Raman spectroscopy
Raman spectra is a kind of scattering spectrum, a means of detecting molecular structure. Information on molecular vibration and rotation can be obtained by analyzing the scattering spectrum with a different frequency than the incident light.

Raman effect: originates from molecular vibration (and lattice vibration) and rotation, so knowledge of molecular vibration energy levels (lattice vibration energy levels) and rotational energy level structures can be obtained from the Raman spectrum. The Raman effect is the result of the interaction between photons and optical phonons.
Raman test: Raman spectrum can test material composition, tension and stress, crystal symmetry and orientation, crystal quality, total amount of material, information on material functional groups, etc.
§2 The principles of Raman spectroscopy
There are three relative interactions between matter and light: reflection, scattering and transmission. According to these three situations, corresponding spectral detection methods were derived (see the table below), and Raman spectroscopy came into being.
| Emitted light | Atomic emission spectroscopy (AES), atomic fluorescence spectroscopy (AFS emission spectroscopy), X-ray fluorescence spectrometry (XFS), molecular fluorescence spectrometry (MFS), etc. |
| Absorption spectroscopy | Ultraviolet-visible light method (UV-Vis), atomic absorption spectroscopy (absorption spectroscopy AAS), infrared spectroscopy (IR), nuclear magnetic resonance (NMR), etc. |
| Combined Scattering | Raman Scattering Spectroscopy (Raman). |
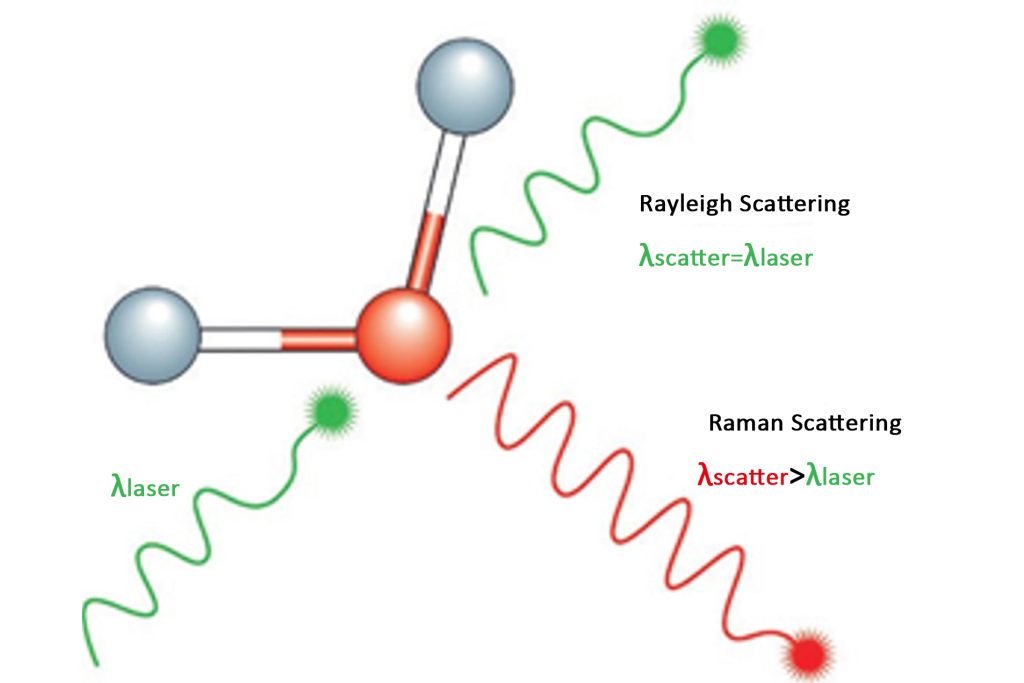
When light strikes a substance, elastic scattering and inelastic scattering occur. The scattered light of elastic scattering has the same component as the wavelength of the excitation light. The scattered light of inelastic scattering has components that are longer and shorter than the wavelength of the excitation light. This is collectively called the Raman effect.
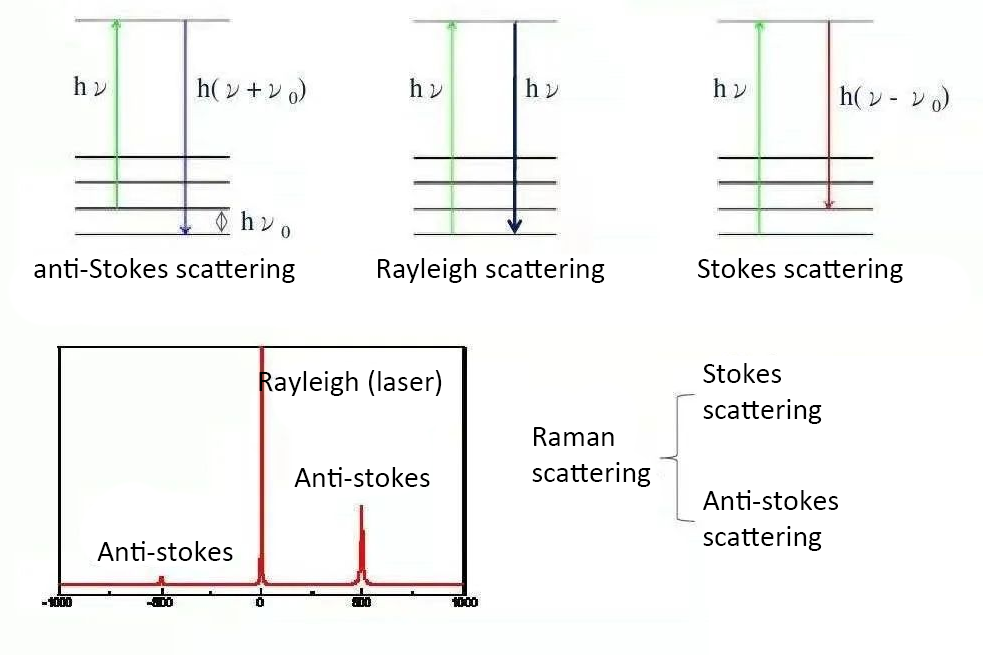
In the scattering spectrum of transparent media, the component with the same frequency as the incident light frequency υ0 is called Rayleigh scattering; Raman scattering is divided into Stokes scattering and Anti-Stokes scattering and the frequency is symmetrically distributed on the spectral lines or bands υ0 on both sides of υ0 ±υ1 is the Raman spectrum, in which the smaller frequency components υ0-υ1 are also called Stokes lines, and the higher frequency components υ0+υ1 are also called anti-Stokes lines.
| Rayleigh Scattering | The scattering intensity with the same frequency as the incident light is about 10-3 times that of the incident light |
| Raman scattering | The scattering intensity that is different from the incident light frequency is about 10-6~10-8 times that of the incident light |
| Stokes line | The frequency of scattered light is less than the frequency of incident light |
| Anti-Stokes line | The frequency of scattered light is greater than the frequency of incident light |
| Raman shift | Difference between scattered light frequency and excitation light frequency Δv=IV0-VSI |
Table 2 Noun distinctions related to Raman
Commonly detected Raman experiments are Stokes scattering, and the difference in frequency between Raman scattered light and Rayleigh light is called Raman shift. The abscissa represents the Raman shift, and the ordinate represents the Raman light intensity.
Raman shift has nothing to do with the frequency of incident light but is only related to the vibration and rotational energy levels of material molecules. Different material molecules have different vibrational and rotational energy levels and have specific Raman shifts. Therefore, Raman can be used to identify the structure of matter. analysis and research.
§3 Three types of Raman imaging
- Microscopic area Raman for single-point imaging;
- Raman for surface imaging of a certain area;
- Confocal Raman can be imaged by observing different depths in the XYZ direction. Most commercial instruments for Raman imaging are combined with optical microscopes, and multiple imaging spectra can be obtained from one sample.
§4 Raman scattering spectrum has the following three obvious characteristics:
- Although the wave number of the Raman scattering line varies with the wave number of the incident light, for the same sample, the displacement of the same Raman line has nothing to do with the wavelength of the incident light, but is only related to the vibration and rotation energy level of the sample;
- In the Raman spectrum with wave number as the variable, Stokes lines and anti-Stokes lines are symmetrically distributed on both sides of the Rayleigh scattering line. This is because in the above two cases, respectively, we get Or a vibrational quantum of energy is lost.
- Generally, the Stokes line is stronger than the anti-Stokes line. This is due to the Boltzmann distribution, where the number of particles in the vibrational ground state is much larger than the number of particles in the vibrational excited state.
§5 Six major differences between laser Raman spectroscopy and infrared spectroscopy
Both Raman spectroscopy and infrared spectroscopy can obtain information about various normal vibration frequencies and related vibration energy levels inside the molecule, which can be used to identify the functional groups present in the molecule. The two have many differences and similarities.
Comparison of Raman spectroscopy and infrared spectroscopy:
- In essence, both are vibrational spectra, and they measure the excitation or absorption of the ground state, and the energy range is the same. However, infrared spectroscopy measures the absorption of light, and the abscissa is expressed by wave number or wavelength, while Raman spectroscopy measures the scattering of light, and the abscissa is the Raman shift.
- IR is easy to measure and the signal is very good, while the signal of Raman is very weak.
- The wavelength range used is different. IR uses infrared light, especially mid-infrared, which many optical materials cannot penetrate, which limits its use. Raman has many wavelengths to choose from, ranging from visible light to NIR. The incident light and detection light of the infrared spectrum are both infrared light, while the incident light of the Raman spectrum is both visible light and infrared light, and the scattered light is also both visible light and infrared light.
- In terms of sample preparation, IR is sometimes relatively complicated, time-consuming, and may damage the sample, but Raman does not have these problems.
- Some vibrations can be detected by both infrared and Raman, and some vibrations can only be detected by one of them. For example, oxygen and nitrogen can only be detected using Raman.
- In the analysis of structural analysis, the focus of the two is different. Infrared spectroscopy focuses on detecting groups, is suitable for polar bonds, and is mostly used to measure organic substances. Raman spectroscopy detects molecular skeletons, is suitable for non-polar bonds, and can test both organic and inorganic substances. Infrared is greatly interfered by water. Some information that cannot be detected in infrared spectroscopy can be well expressed in Raman spectroscopy
Of course, there are some differences and similarities between infrared spectroscopy and Raman spectroscopy that are not listed here.
