In organisms, the intercellular substance and body fluid components within cells constitute the microenvironment for cell survival. A stable microenvironment is the most important condition for maintaining normal cell proliferation, differentiation, metabolism and other functional activities. Information transmission within or between cells is also inseparable from the environment in which they are located.
In addition, in the treatment of some diseases, the interaction between drug molecules and diseased tissue molecules will also change its microenvironment. The effect of drug treatment can be evaluated by studying the microenvironment of cells or tissues.
Therefore, high-resolution and high-sensitivity detection of cellular microenvironment has become an important research topic in biomedicine.
Optical microscopy technology can reveal tiny substances and structures on the micrometer or even nanometer scale in front of us. It is the key to opening the door to the microscopic world and observing cell tissues and biological microenvironments.
If the imaging is based on the fluorescence intensity of fluorescent luminophores for data analysis, it can be called fluorescence intensity microscopy. This type of microscopy technology collects the fluorescence intensity at various positions of the sample through the eyepiece of the microscope, and then the morphology of the biological tissue can be obtained. It has a high imaging spatial resolution but is affected by the concentration of the fluorophore, and its measurement accuracy and quantitative analysis capabilities are affected. not ideal.
Fortunately, among a wide variety of microscopy techniques, fluorescence lifetime imaging microscopy (FLIM) has the ability to carry out high-resolution and high-precision measurements of biological macromolecule structures, dynamic information, and molecular environments, so its importance is increasing day by day. Improved and widely used in fields such as biological research and clinical diagnosis.
§01 What is Fluorescence Lifetime Imaging (FLIM)?
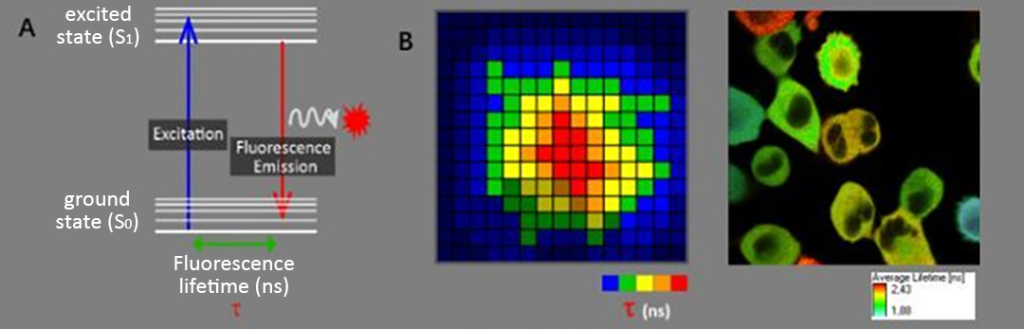
Fluorescence lifetime: Fluorescence refers to the phenomenon that after a fluorescent molecule absorbs energy, its electrons in the ground state (S0) transition to the excited state (S1), and after a short stay, light is released when the excited state (S1) returns to the ground state (S0). , and the time the fluorescent molecule stays in the excited state is the fluorescence lifetime (Figure 1A). Like fluorescence spectrum, fluorescence lifetime is also an intrinsic and unique property of fluorescent substances.
FLIM (Fluorescence Lifetime Imaging): It is a microscopy imaging technology based on fluorescence lifetime. Its imaging results provide lifetime information of pixel locations (as shown in Figure 1B), allowing us to do more besides fluorescence intensity imaging. Perform in-depth functional measurements on your samples. Fluorescence lifetime imaging has many advantages that are different from fluorescence intensity imaging, such as not being affected by factors such as fluorescent substance concentration, photobleaching, and excitation light intensity. However, it will change due to changes in molecular conformation, intermolecular interactions, molecular microenvironment, physiological state, and other conditions. Therefore, fluorescence lifetime can be used to analyze these changes in molecules. FRET-based on fluorescence lifetime measurement is one of the important applications of FLIM.
§02 Why use fluorescence lifetime imaging (FLIM)?
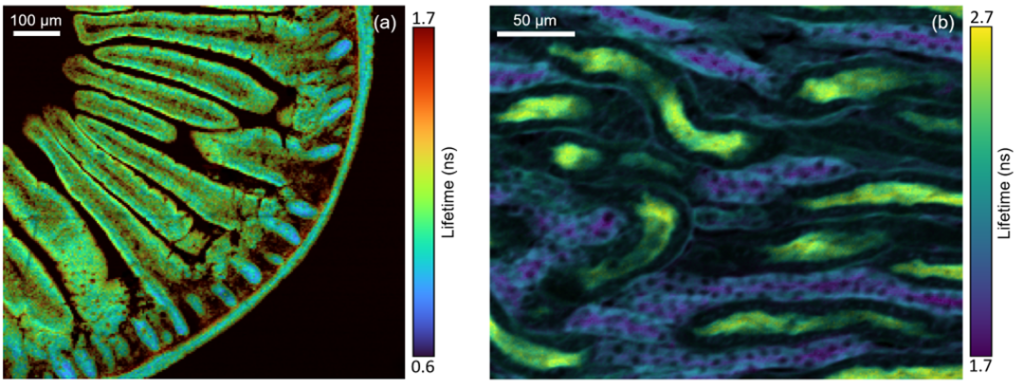
Fluorescence lifetime imaging (FLIM) is an imaging technique in which changes in the fluorescence lifetime of a sample create contrast in the image (Figure 2). FLIM is widely used in biomedical imaging, where tissues and cells are stained with one or more fluorescent dyes. The fluorescence lifetime of dyes depends on the local microenvironment, and FLIM provides additional dimensions such as environmental information than other imaging techniques (such as wide-field imaging). FLIM is also increasingly used to study photoluminescent materials, such as imaging carrier lifetime changes in nanomaterials, solar cells, and semiconductors.
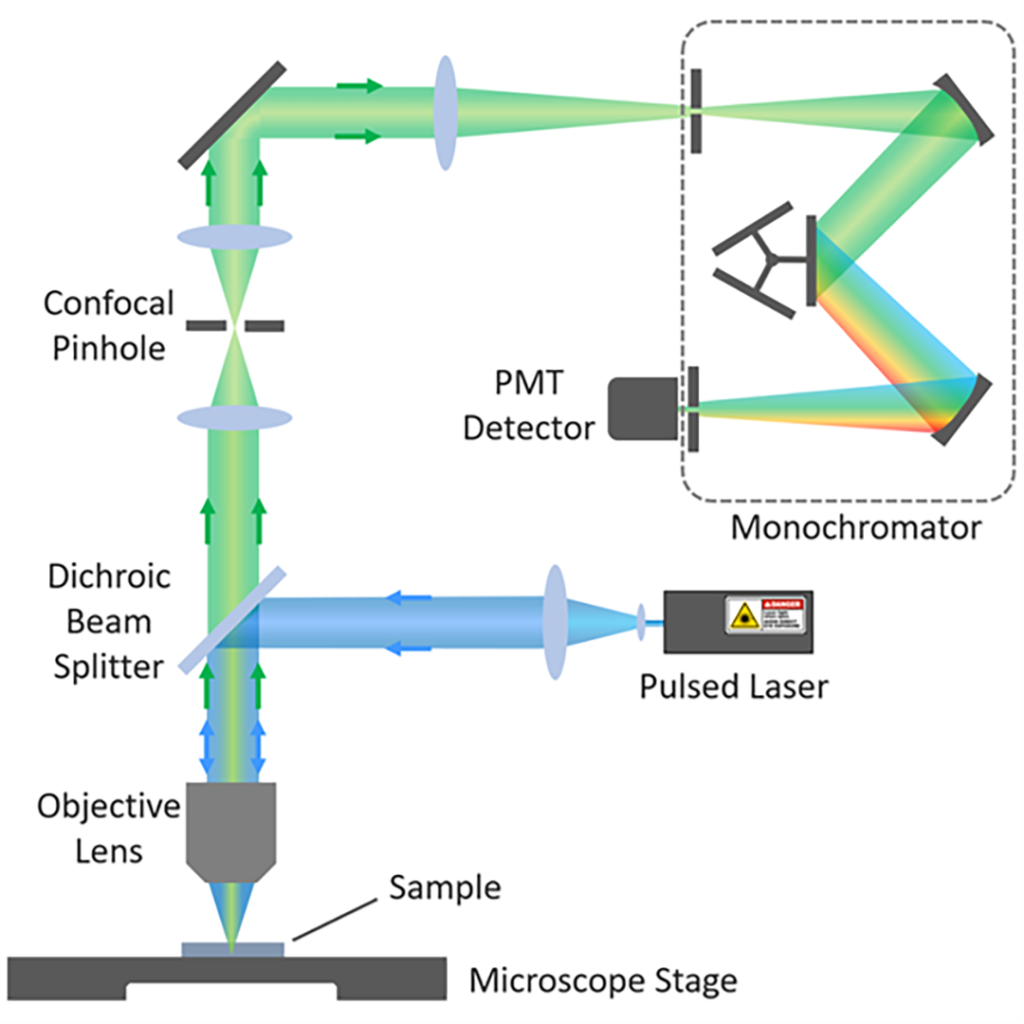
§03 Fluorescence Lifetime Imaging (FLIM) Confocal Microscopy
FLIM imaging was acquired using confocal microscopy (Figure 3). The microscope is equipped with a pulsed laser, which is focused by an objective lens onto the sample to form a small spot. Fluorescence from the sample is selected using a filter or monochromator (arrangement in Figure 3) and detected using a single-photon counting detector such as a photomultiplier tube (PMT).
§04 Fluorescence Lifetime Imaging (FLIM) Acquisition
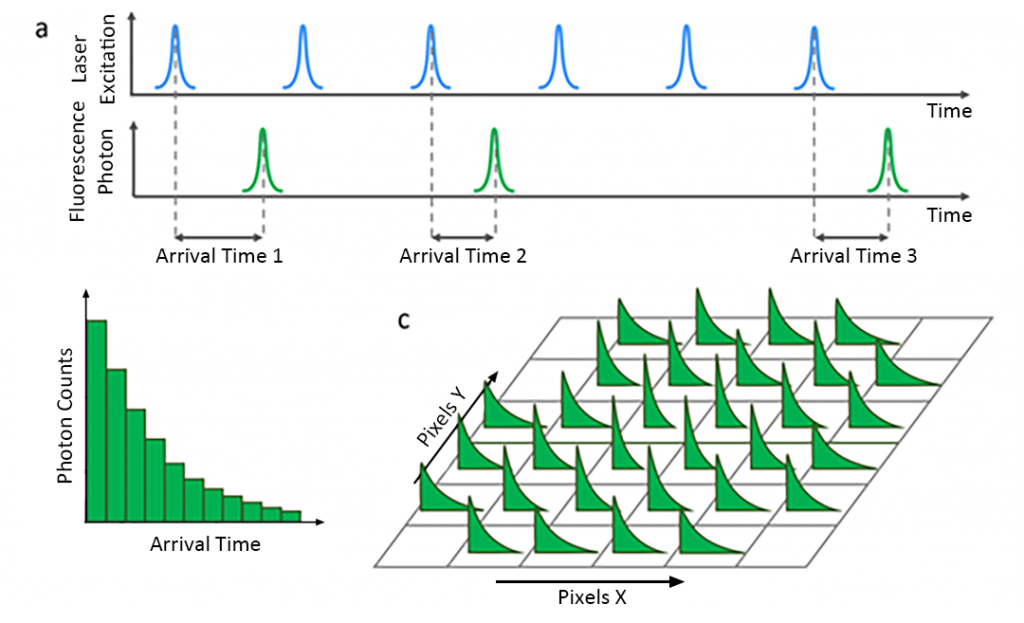
Fluorescence decay was obtained using time-correlated single photon counting (TCSPC). In TCSPC, the sample is excited with a pulsed laser and the time between the laser pulse and the detected fluorescence photon is measured (Figure 4a). This process is repeated millions of times to create a histogram of fluorescence photon counts versus arrival times (Figure 4b).
Divide the area of interest on the sample into pixels and record the fluorescence decay of each pixel using TCSPC (Figure 4c). The laser spot is directed to each pixel by moving the sample stage (stage scanning) or the laser spot (laser scanning). This process occurs sequentially, recording the fluorescence decay of each pixel in turn to build a collection of histogram data.
§05 Fluorescence Lifetime Imaging (FLIM) Analysis
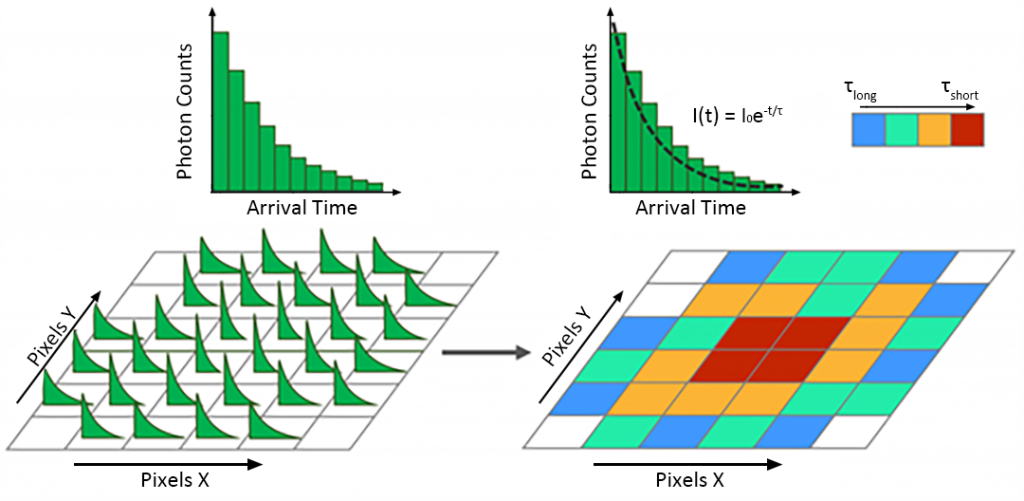
Calculate the fluorescence lifetime of each pixel’s fluorescence decay histogram. It is usually calculated by fitting a single exponential model to each decay using the least squares method to obtain the fluorescence lifetime τ (Fig. 5). The fluorescence lifetime obtained by fitting is mapped to the color map to construct a color-coded fluorescence lifetime image.
§06 Conclusion
Fluorescence lifetime imaging (FLIM) complements photoluminescence spectroscopy and provides information about the sample microenvironment. Time-correlated single photon counting (TCSPC) was used to obtain lifetime information, and a single exponential model was used to fit the fluorescence decay curve to construct a color-coded fluorescence lifetime image. The RMS1000 confocal micro-Raman spectrometer can not only obtain Raman spectrum information, but also realize fluorescence lifetime imaging (FLIM), realize all-round multi-dimensional sample information detection, and meet the multi-dimensional needs of scientific research.