Since the late 16th century, scientists have been using optical microscopes to explore the complex microscopic world of organisms. However, due to the limitation of the optical diffraction limit, traditional optical microscopy has a lateral resolution of about 200 nm and an axial resolution of 500 nm, making it impossible to observe smaller biomolecules and structures. Breaking through the optical diffraction limit has always been the dream and goal of scientists.
However, with the emergence of technologies such as scanning electron microscopy, scanning tunneling microscopy and atomic force microscopy, it has become possible to achieve nanometer-level resolution. However, these technologies have the disadvantages of being highly destructive to samples and can only observe the surface, making them unsuitable for biology. Observation of samples, especially living samples.
In the past decade or so, a series of super-resolution imaging technologies suitable for imaging biological samples have emerged, including structured light illumination (SIM), stimulated emission depletion fluorescence microscopy (STED), photoactivated localization microscopy (PALM), Stochastic Optical Reconstruction Microscopy (STORM) and more.
Optical resolution limit
Light propagates in the form of waves. When a point light source is focused into a small light spot on the imaging surface through a lens, no matter how good the objective lens is, the imaging light spot will be larger than the actual luminous point. This is because light waves are diffracted at the edge of the objective diaphragm, spreading the wavefront outward (Figure 1).
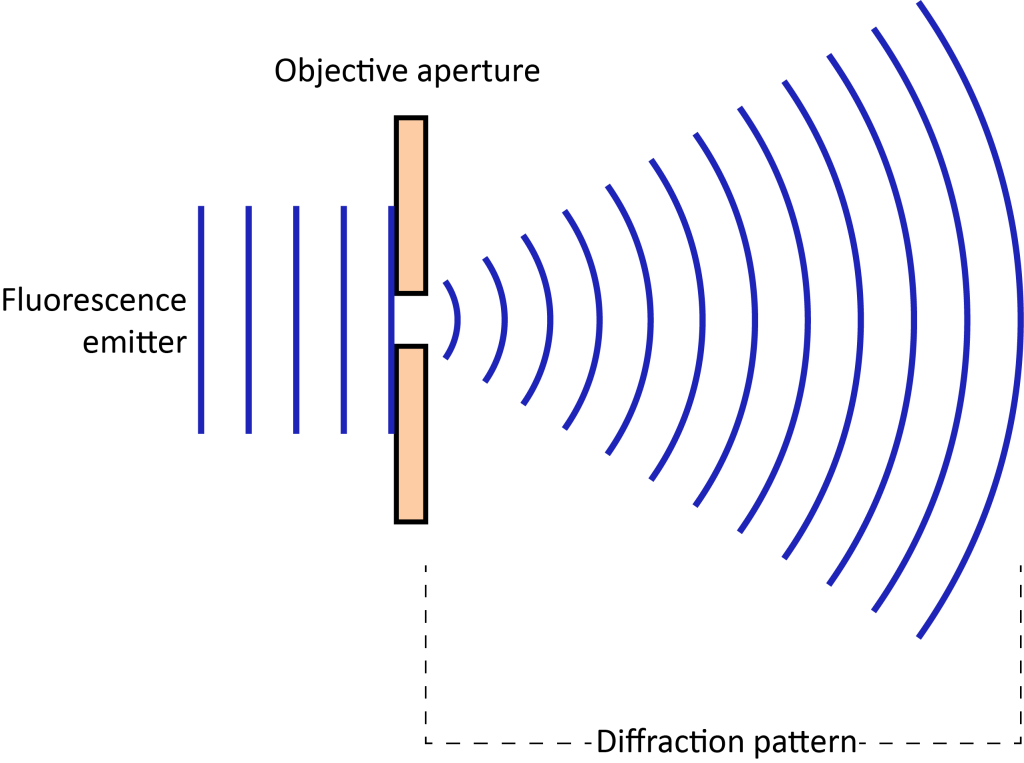
The diffraction spot has a bright central point and a series of diffraction rings of decreasing brightness surrounding it, called the Airy disk. When two points are too close, the resulting image spots overlap and the images of the two points become indistinguishable. Therefore, there is a limit to optical resolution. According to the Rayleigh criterion, when the center of one Airy disk coincides with the first-level dark ring of another Airy disk, the image formed by the two points can just be distinguished (Figure 2). The formula is expressed as follows:
d = 1.22λ/2NA
d represents the resolution, λ is the wavelength of the emitted light, and NA is the objective numerical aperture.
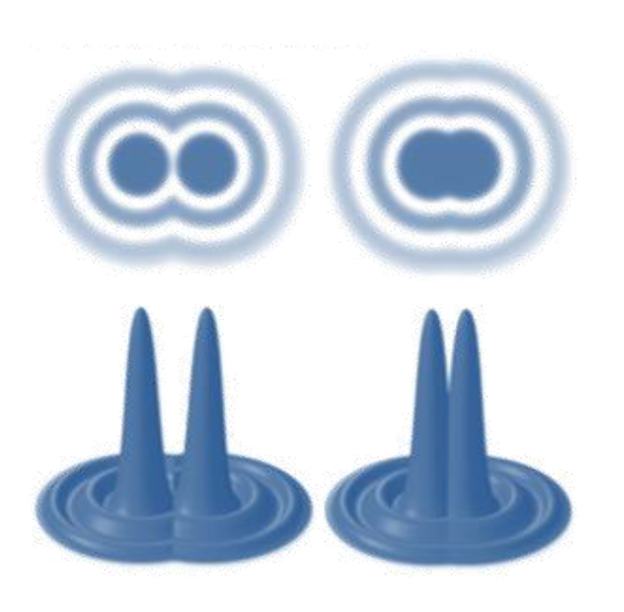
Taking GFP as an example, λ=510nm, using an objective lens of NA=1.4, the limit of optical resolution is 222nm. But in biological systems, subcellular structures such as membrane proteins, transporters, and ribosomes tend to be smaller than 50 nm and are often not far enough apart. This makes it impossible to directly image it clearly.
How to do it? Of course, the pace of scientific research cannot stop here. To overcome the diffraction limit, researchers have developed a series of super-resolution imaging methods. This article will introduce super-resolution microscopy imaging technology.
1. What is super-resolution microscopy?
The super-resolution microscope is an analytical instrument used in the field of basic medicine. The super-resolution microscope (Figure 3) is an analytical instrument used in the field of basic medicine, including a confocal scanning head, a high-sensitivity EMCCD detector, and a laser coupling instrument, a synchronization controller, laser dynamics module (FRAP), research-grade inverted fluorescence motorized microscope, super-resolution system-stochastic optical reconstruction system, high-precision motorized XY platform and high-speed piezoelectric ceramic Z-axis, living cell culture device, image Workstation and acquisition control software. Super-resolution microscopy can break through the resolution limit of traditional optical microscopes and achieve high-resolution imaging at the nanometer scale. These include stimulated emission extinction microscopy (STED) and single molecule fluorescence microscopy (SMLM).
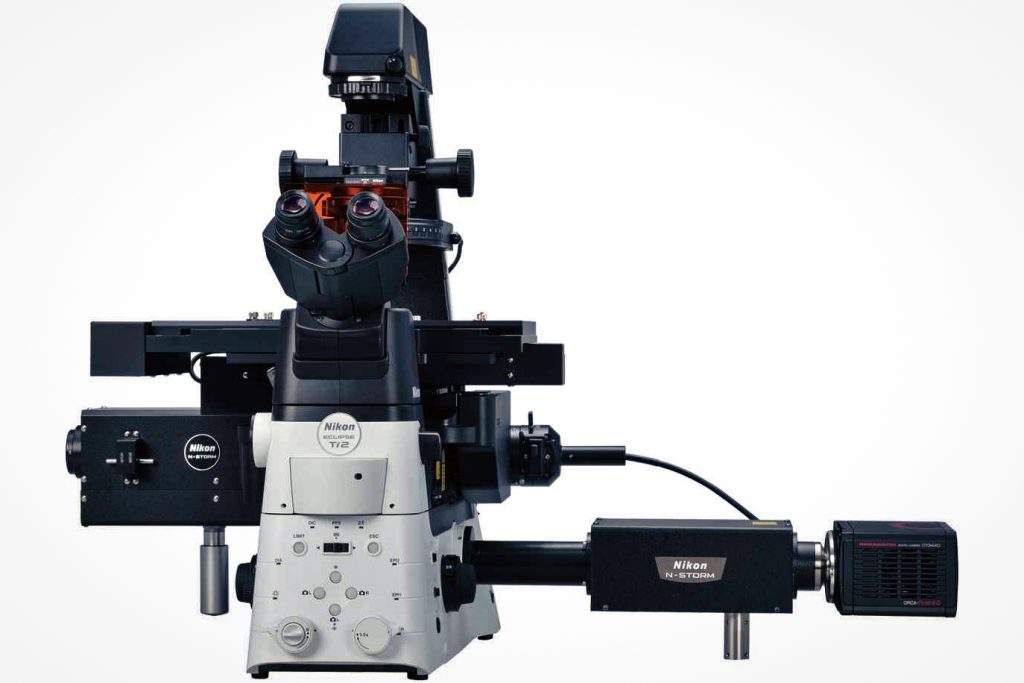
2. Working principle of super-resolution microscope
The working principle of super-resolution microscopy can be illustrated by two common techniques: stimulated emission extinction microscopy (STED) (Figure 4) and single-molecule fluorescence microscopy (SMLM) (Figure 5)
(1) Principle of Stimulus Emission Extinction Microscopy (STED)
STED microscopes use laser beams to excite fluorescent markers in the sample and achieve super-resolution imaging by selectively extinguishing the emission signals of the fluorescent markers. Here’s how it works:
- a.STED microscopes use two laser beams: a stimulation laser beam and a STED laser beam.
- b. The stimulation laser beam is used to excite the fluorescent markers in the sample, causing them to emit light.
- c. The STED laser beam overlaps in time and space with the stimulation laser beam, but has an opposite phase.
- d.STED laser beam uses nonlinear optical effects to suppress fluorescence emission in a specific area so that it is not captured by the detector.
- e. By controlling the shape and intensity of the STED laser beam, high-resolution imaging of fluorescent markers can be achieved, breaking through the resolution limit of traditional optical microscopes.
(2) Principle of single-molecule fluorescence microscopy (SMLM)
SMLM utilizes the continuous imaging and localization of individual fluorescently labeled molecules in time and space and then reconstructs this localization information into high-resolution images. Here’s how it works:
- a. The fluorescently labeled molecules in the sample are excited and emit light signals, appearing and extinguishing randomly in a short period of time.
- b. During the imaging process, only a small part of the fluorescent molecules are in the excitation and emission states, and the position information of these fluorescent molecules is collected.
- c. This continuous excitation and detection process is repeated multiple times to collect a large amount of position information.
- d. Finally, statistical algorithms are used to analyze and reconstruct these positions to obtain high-resolution images.
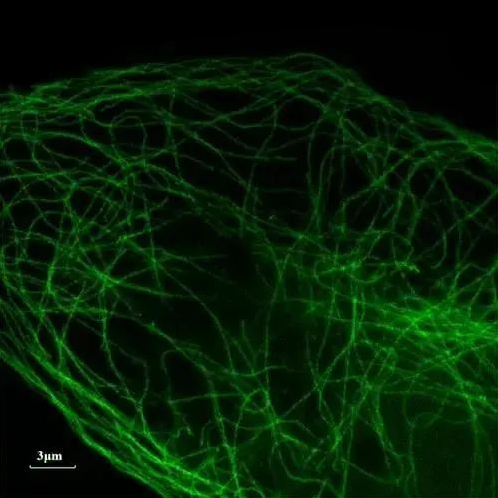
3. Types of super-resolution microscopes
There are many types of super-resolution microscopy, here are six common types:
● Structured Illumination Microscopy (SIM)
Illuminating the sample using a structured beam, combined with imaging and computational methods, makes it possible to obtain super-resolution images.
● Stimulated Emission Depletion Microscopy (STED)
A laser beam is used to excite fluorescent markers in the sample, and the fluorescence emission is controlled and suppressed by stimulating and extinguishing the beam to obtain super-resolution images.
● Single-Molecule Localization Microscopy (SMLM)
By continuously exciting and detecting the position of individual fluorescently labeled molecules, and finally stitching these positional information together, a super-resolution image is obtained.
● Transient Absorption Microscopy (TAM)
Super-resolution imaging is achieved by exciting the sample with laser pulses and measuring changes in light absorption by the sample.
● Near-Field Scanning Optical Microscopy (NSOM)
Taking advantage of extremely close light-sample interactions, super-resolution images are achieved by collecting light signals from the probe tip.
● Light Sheet Microscopy (LSM)
By vertically irradiating thin slices of the sample, the sample is divided into thin light segments, reducing light scattering and phototoxicity, and achieving high-resolution, high-contrast imaging.
4. Application scenarios of super-resolution microscopes
Super-resolution microscopy has a wide range of application scenarios in the field of basic medicine. It can provide high-resolution image information and reveal the details of microstructure and function in cells and tissues. The following are some application scenarios of super-resolution microscopy in the medical field:
- a. Cell biology research
Super-resolution microscopy can perform high-resolution imaging of cellular structures such as organelles, cytoskeleton, proteins, and DNA, which helps to understand various biological processes within cells, such as cell division, cell transport, and membrane protein movement.
- b.Cancer research
Super-resolution microscopy can observe the morphological changes, intracellular signaling and molecular interactions of cancer cells, providing important information for cancer research and diagnosis. Imaging and intracellular tracking of cancer-derived exosomes by super-resolution microscopy (Figure 6).
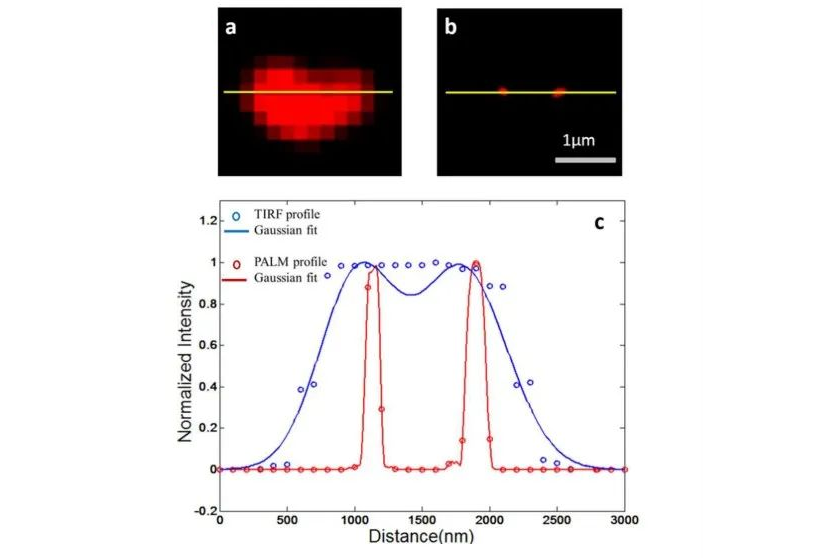
- c. Neuroscience research
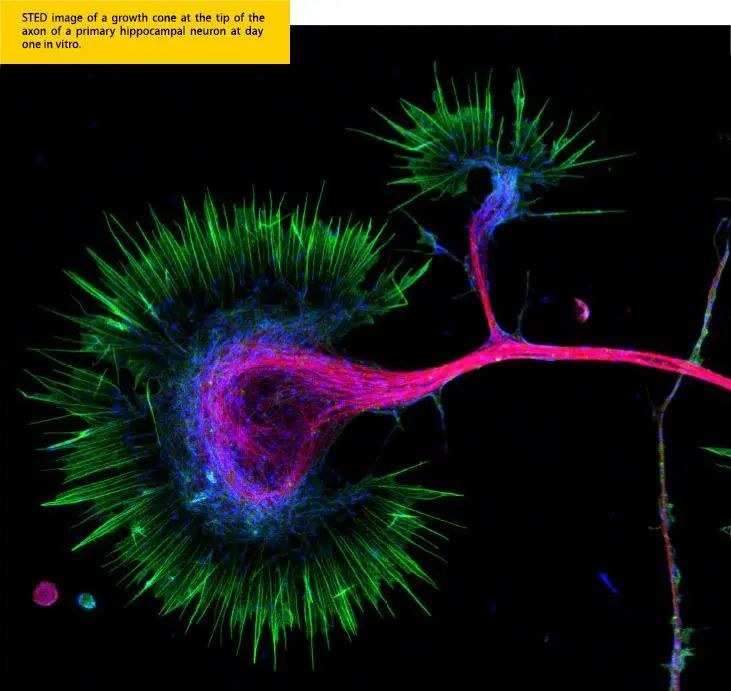
Super-resolution microscopy can reveal the cellular structure, synaptic connections, neurotransmitter release and other processes of neurons, helping us understand the functions of the nervous system and disease mechanisms, such as neurodegenerative diseases and neurodevelopmental disorders.
- e.Immunology research
Super-resolution microscopy can observe immune processes such as immune cells, antigen-antibody interactions, and immune complex formation, which can help to gain a deeper understanding of the regulation of the immune system and the mechanisms of disease occurrence.
- f.Tissue engineering and regenerative medicine
Super-resolution microscopy can perform high-resolution imaging of biological materials, artificial tissues and regenerative medicine organs, evaluate their structure and function, and provide support for the development of tissue engineering and regenerative medicine.
- g. Drug research and efficacy evaluation
Super-resolution microscopy can observe the dynamic distribution and interactions of drug molecules in cells and tissues, helping to optimize drug development and efficacy evaluation.
In short, super-resolution microscopy plays an important role in basic medical research, helping to expand our understanding of biological cells, tissues and molecules, deepen our understanding of disease mechanisms, and promote the progress of medical science.