Fluorescence Phenomenon
Fluorescence refers to the process where fluorescent substances emit longer wavelength light almost simultaneously upon irradiation with specific wavelength light (Figure 1). When a molecule (such as those within a fluorescent group) is irradiated with light of a specific wavelength (excitation wavelength), the photon energy is absorbed by the electrons of that molecule. Subsequently, the electrons transition from the ground state (S0) to a higher energy level, the excited state (S1′). This process is known as excitation①. The electron remains in the excited state for 10-9–10-8 seconds, during which it loses some energy②. As the electron returns from the excited state (S1) to the ground state, it releases the remaining energy absorbed during the excitation process ③.
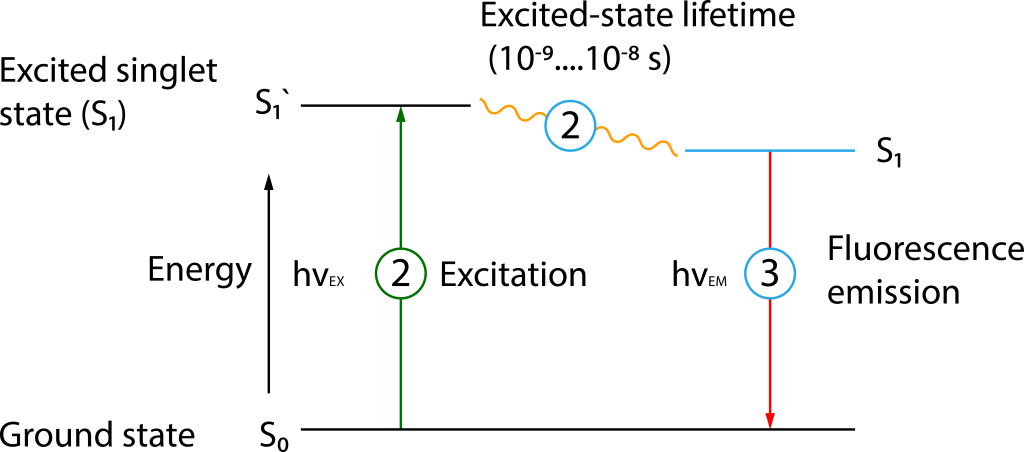
The duration of time a fluorescent molecule spends in the excited state is known as the fluorescence lifetime, typically on the nanosecond scale, which is an inherent property of the fluorescent molecule. Fluorescence Lifetime Imaging (FLIM), a technique that utilizes fluorescence lifetime for imaging, allows for more in-depth functional measurements beyond fluorescence intensity imaging. It provides insights into molecular conformation, molecular interactions, and the microenvironment of molecules that are difficult to obtain with conventional optical imaging techniques.
Another important characteristic of fluorescence is Stokes shift, which refers to the difference in wavelength between the excitation peak and the emission peak (Figure 2). Typically, the emitted light has a longer wavelength compared to the excitation light. This is because, after the excitation of the fluorescent substance and before the emission of photons, the electrons undergo relaxation processes, leading to the loss of some energy. Fluorescent substances with larger Stokes shifts are more suitable for observation under fluorescence microscopes.
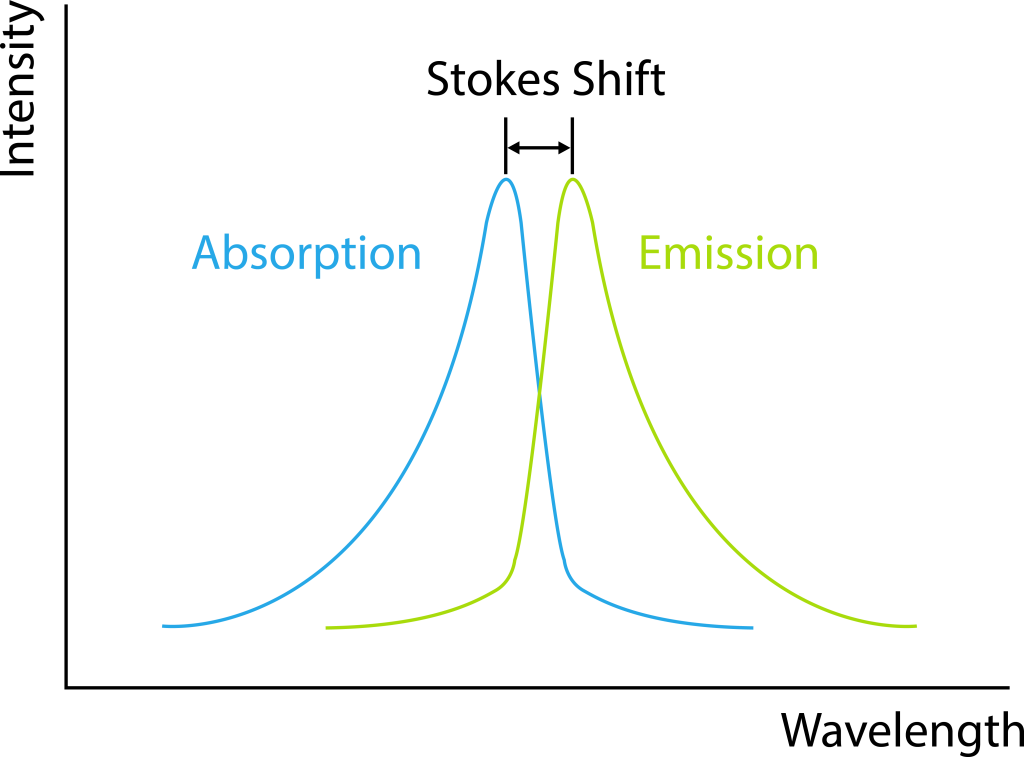
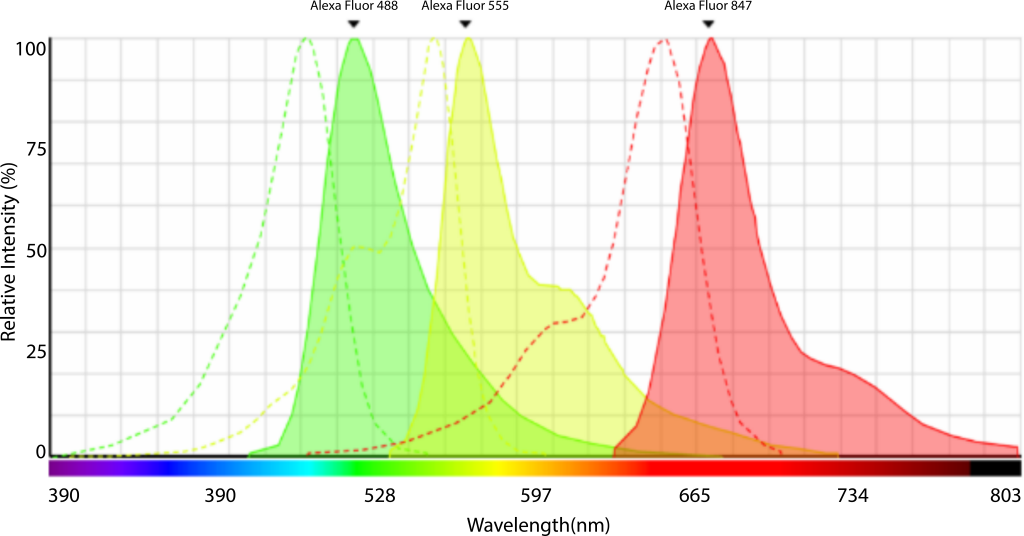
Figure 2: Stokes Shift
Fluorescence Microscopy and Fluorescence Filters
Fluorescence microscopy utilizes fluorescence properties for observation and imaging and finds wide applications in various fields such as cell biology, neuroscience, botany, microbiology, pathology, and genetics. Fluorescence imaging offers high sensitivity and specificity, making it ideal for observing the distribution of specific proteins, organelles, co-localization studies, and interactions, and tracking dynamic processes such as changes in ion concentrations within tissues and cells.
Since most molecules within cells do not fluoresce, fluorescence labeling is necessary for their observation. There are numerous methods for fluorescent labeling, including direct labeling (e.g., using DAPI for DNA labeling), immunostaining using the antibody-antigen binding properties, tagging target proteins with fluorescent proteins (e.g., GFP, green fluorescent protein), or using reversibly binding synthetic dyes (e.g., Fura-2).
Figure 3: Inverted Fluorescence Microscope and Filter Wheel
Fluorescence microscopes are now standard imaging equipment in various laboratories and imaging platforms, serving as valuable tools in daily experiments. They are broadly categorized into three types: upright fluorescence microscopes (suitable for tissue sections), inverted fluorescence microscopes (suitable for live cells while also accommodating tissue sections), and fluorescence stereomicroscopes (suitable for larger specimens such as plants, zebrafish (adult/embryonic), Drosophila, and rodent organs).
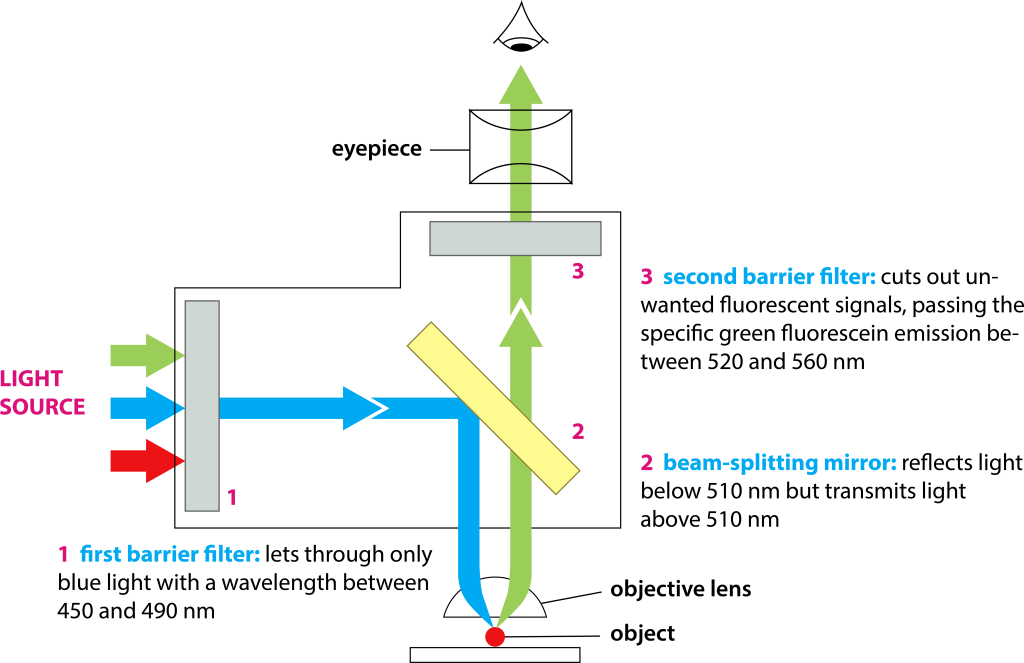
Fluorescence filters are essential components of fluorescence imaging systems, consisting of excitation filters, emission filters, and dichroic mirrors, which are installed on filter wheels. The number of positions in filter wheels may vary among different microscopes, and some microscopes use filter sliders.
Filters play a crucial role in fluorescence imaging: the excitation filter selects excitation light to excite the sample while blocking light of other wavelengths. The light passing through the excitation filter goes through the dichroic mirror (which reflects excitation light and transmits fluorescence), reflects back through the objective lens for focusing onto the sample, excites the corresponding fluorescence, and the emitted light is collected by the objective lens. It then passes through the dichroic mirror to reach the emission filter. In Figure 4, for example, the excitation wavelength is 450-490nm, the dichroic mirror reflects light shorter than 510nm, and transmits light longer than 510nm, and the emission light is within the range of 520-560nm.
Commonly used fluorescence filters can be categorized into two types: long-pass (LP) and band-pass (BP) filters. Band-pass filters are typically defined by their center wavelength and bandwidth, such as 480/40 indicating light within the range of 460-500nm. Long-pass filters like 515 LP transmit light longer than 515nm (Figure 5).
Figure 5: FITC Spectrum Curve and Filters
Fluorescent substances have characteristic excitation (absorption) and emission spectra, where the excitation peak represents the optimal excitation wavelength (highest excitation efficiency, thereby reducing excitation light energy to protect cells and dyes), and the emission spectrum indicates the range of emitted fluorescence wavelengths. Hence, in experiments, we strive to choose wavelengths closest to the excitation peak for excitation while ensuring that the receiving range includes the emission peak. For instance, Alexa Fluor 488 has an excitation peak at 500nm, and a suitable excitation filter for fluorescence microscopy would be 480/40.
Figure 6: Alexa Fluor 488 Spectrum Curve
Detailed information about filters can be found in microscopy imaging software. Understanding dyes and finding the most suitable filters for samples are crucial for fluorescence imaging. The spectral information of fluorescent dyes and fluorescent proteins is usually indicated in the manuals or can be searched online.
In filter selection, considerations extend beyond excitation and emission wavelengths of fluorescent probes. For multi-color labeled samples, it is necessary to consider whether there is nonspecific excitation and whether there is spectral overlap. Additionally, the choice of fluorescent light source is essential. Commonly used fluorescent light sources include mercury lamps, metal halide lamps, and the rapidly evolving LED light sources. The spectral characteristics of fluorescent light sources can be continuous or discontinuous, and the energy varies in different bands. LED light sources are gradually becoming the primary light source for fluorescence microscopes due to their relatively narrow spectral bandwidth, stable energy output, ultra-long lifespan, and environmental safety.
In addition to built-in filters in microscopes, there are external rapid filter wheels (Figure 7), which allow for fast filter switching with adjacent positions on fluorescence microscopes, achieving high-speed multi-color experiments such as FRET and Fura-2 ratiometric calcium imaging (Figure 8).
Figure 7: External Fast Wheel (EFW) of Fluorescence Microscope
Figure 8: Calcium Imaging, Cultured hippocampal astrocytes from 18-day-old embryos of Sprague-Dawley rats. Courtesy of: Drs. Kazunori Kanemaru and Masamitsu Iino, Department of Pharmacology, Graduate School of Medicine, The University of Tokyo
Diverse Fluorescence Microscopy Imaging Techniques
To meet various fluorescence imaging needs, in addition to fluorescence microscopes, various fluorescence microscopy imaging solutions have been developed:
- Wide-field high-definition imaging systems effectively eliminate out-of-focus interference signals during imaging, presenting clear images while offering high-speed imaging.
- Confocal laser scanning microscopes utilize pinholes to exclude out-of-focus interference, achieving optical sectioning, and obtaining high-definition images, and three-dimensional stereoscopic images.
- Super-resolution microscopes and nano microscopes that surpass the diffraction limit enable observation of fine structures smaller than 200nm.
- Multiphoton imaging systems utilize the principle of multiphoton excitation for thick tissue and in vivo deep imaging.
- Light sheet imaging technology with high spatiotemporal resolution features fast imaging speed, high resolution, and low phototoxicity, and is particularly suitable for developmental studies and in vivo dynamic observations.
- Fluorescence Lifetime Imaging (FLIM), unaffected by fluorescent substance concentration, photobleaching, and excitation light intensity, enables more in-depth functional precise measurements.
- Fluorescence Correlation Spectroscopy (FCS) and Fluorescence Cross-Correlation Spectroscopy (FCCS) measure the number of fluorescent molecules and diffusion coefficients, thereby analyzing molecular concentrations, molecular sizes, viscosity, molecular motion, molecular binding/dissociation, and optical properties of molecules.
- Total Internal Reflection Fluorescence Microscopy (TIRF) provides extremely high z-axis resolution, making it ideal for studying molecular structures and dynamics on cell membrane surfaces.
Fluorescence microscopy imaging technology is widely applied, with a variety of types available, and new technologies continue to emerge. Researchers can choose the most suitable technology to accomplish their research goals.